Along with a change in season, pharmacists should gear up for a raft of changes set to take place on 1 March 2024.
From policy updates, scope of practice announcements to the Eighth Community Pharmacy Agreement – here’s all the impending changes you need to know about.
1. Tranche 2 of 60-day dispensing begins
As the second stage of 60-day dispensing kicks off, pharmacists should familiarise themselves with the new medicines that will be added to the list.
More Pharmaceutical Benefits Scheme (PBS) listed products will be added to the list of eligible 60-day dispensing medicines from 1 March 2024 – including medicines prescribed for chronic conditions such as diabetes, epilepsy and autoimmune disorders.
While the full list of medicines is not yet publicly available, pharmacists can find the second tranche PBS medicines and current item codes on the Department of Health and Aged Care website on 1 March.
The third and final 60-day dispensing tranche will come into effect on 1 September 2024.
2. 605 medicines drop off the Continued Dispensing formulary
On leap day (29 February 2024), the National Health (Continued Dispensing – Emergency Measure) Determination 2023 was scheduled to lapse. However, the instrument was extended late on 29 February to have a new expiration date of 31 March.
This means most PBS medicines can continue to be supplied via Continued Dispensing for an extra month.
While is good news for patients and pharmacists in most jurisdictions, in others pharmacists are still only able to dispense the 150 molecules listed in the National Health (Continued Dispensing) Determination 2022:
- New South Wales, where the emergency measure was never adopted
- Victoria, where Public Health Emergency Order 25 enabling expanded Continued Dispensing expired on 6 February 2024.
The PSA regulation hub has been updated to reflect these changes.
3. No more imported vapes?
The next step of the federal government’s vaping reforms will come into effect on Thursday, banning the importation of all vapes, irrespective of nicotine content, for commercial or personal use.
At a press conference today (28 February 2024) on vaping reforms, among other things, federal Minister for Health and Aged Care Mark Butler seemed confident in the border force’s ability to uphold the job.
‘The Australian Border Force – and the TGA have, since the 1 of January been provided with extra resources to do our job, which is to stop these things coming in over the border in the first place,’ he said.
Should border control stem the flow of vapes into Australia, pharmacists should brace for an increase in demand for therapeutic vaping products on prescriptions and associated patient counselling.
For more information, pharmacists can refer to:
- the TGA’s Reforms to the regulation of vapes page
- PSA’s Clearing the Air – Navigating the Vaping Reforms with Confidence recorded webinar.
4. 8CPA deadline
Minister Butler set the deadline of 1 March 2024 for 8CPA negotiations last August after bringing negotiations forward by 12 months.
Both the Department of Health and the federal government said in Senate Estimates that they are still working toward a 1 March 2024 agreement start date.
At today’s press conference, Minister Butler said the 8CPA negotiating teams met this morning, with all keen on ‘a deal’.
‘But this is complex material. We are working through it,’ he said.
5. Pilot for skin conditions commences in Victoria
From next month, trained Victorian pharmacists will be able to prescribe medicines for two skin conditions under the Victorian Community Pharmacist Statewide Pilot.
The two skin conditions pharmacists can assess and treat are:
- Herpes zoster (shingles)
- a flare-up of mild plaque psoriasis.
Pharmacists who opt for the skin clinical stream must offer both services. Management Protocols for the two skin conditions are available here. PSA’s training module Mild Plaque Psoriasis and Herpes zoster (shingles) | VIC is now live.
This article was updated to reflect an extension to the National Health (Continued Dispensing – Emergency Measure) Determination 2023 that was annouced on 29 February 2024.




 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.

 Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4
Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4  C – Comorbidity and risk factor management
C – Comorbidity and risk factor management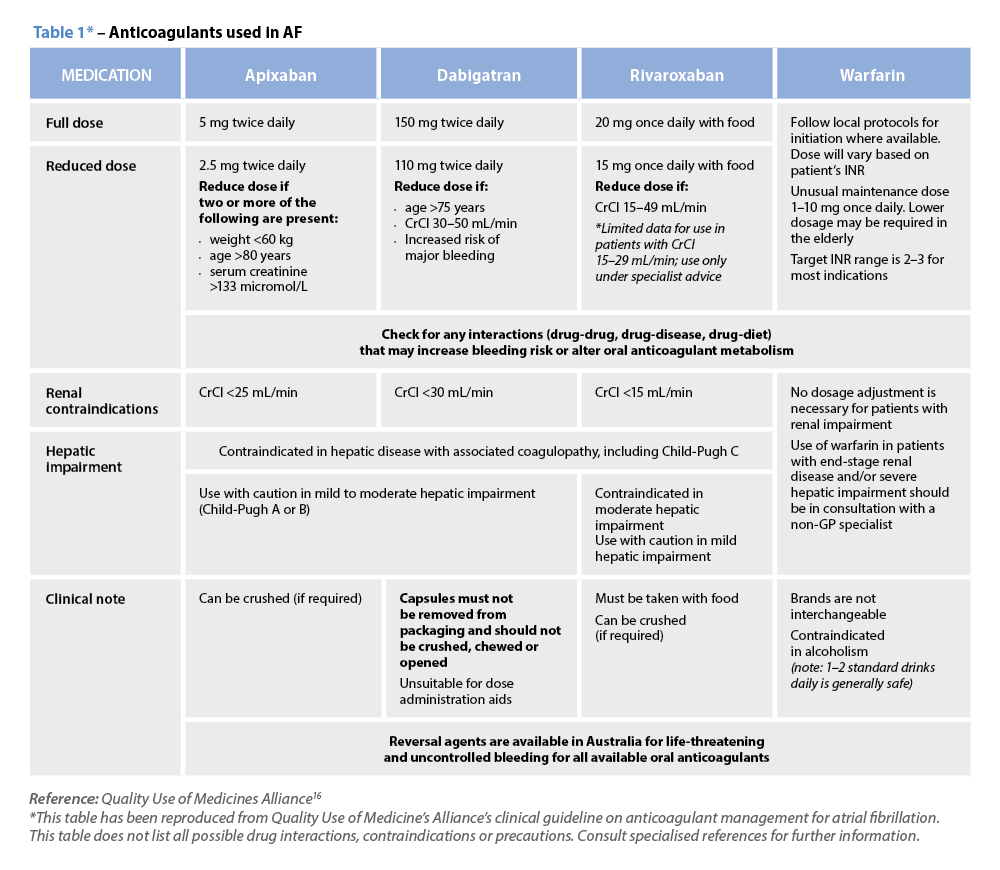 Warfarin
Warfarin




