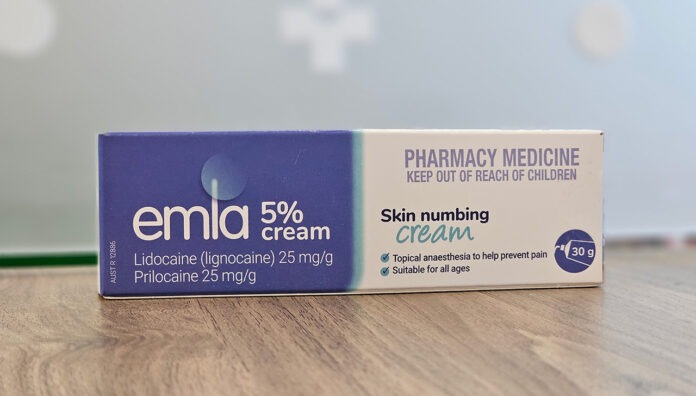
Serious adverse events in infants prompted the Therapeutic Goods Administration (TGA) to issue a safety alert about prilocaine/lidocaine cream.
The anaesthetic cream, sold under the brand name Emla and various other generics, is typically used for topical anaesthesia of the skin before various minor procedures. It is commonly used prior to circumcision, insertion of catheters and vaccination.
What are the signs and symptoms of overdose?
Prilocaine/lidocaine overdose can lead to methaemoglobinaemia, disrupting haemoglobin’s capacity to carry oxygen. Common symptoms include:
- headache
- dizziness
- shortness of breath
- nausea
- poor muscle coordination
- cyanosis.
The complications can be serious, resulting in seizures, heart arrhythmia and death when severe.
What did the TGA reports relate to?
In October 2024, the TGA received two serious adverse event reports following the topical use of Emla in circumcision procedures. Two other incidents of methaemoglobinaemia were also reported in infants.
Following a TGA signal investigation, both serious adverse event cases were suspected to result from an overdose with the local anaesthetic cream.
One case concerned a 3-week-old boy who experienced a seizure after receiving 3–4 g of Emla applied to the penile shaft. He had not been given any other medicines and required supportive treatment in hospital. In another case, also involving a 3-week-old boy, 3 g of Emla was applied before circumcision. The infant developed cyanosis and respiratory distress and was later diagnosed with methaemoglobinaemia.
What do pharmacists need to know?
It’s crucial that pharmacists emphasise to parents and carers the importance of following the instructions for use.
This includes using no more than the recommended amount of prilocaine/lidocaine cream left on the skin for the recommended amount of time. For example, the recommended dose for use prior to circumcision is 1 g of prilocaine/lidocaine cream should be applied to the foreskin for 1 hour.
Pharmacists should communicate reassurance to avoid undue alarm, and reiterate that should the dosing instructions be followed, it is highly unlikely complications should occur – as with many other common medicines, including paracetamol. However, it’s important to break through the misconception that topical medicines can’t lead to adverse events when used in excessive quantities.
Pharmacists should also be alert to the symptoms that could indicate toxicity, and refer patients to the emergency department for prompt diagnosis and treatment.
Will there be any change to packaging?
Following the adverse event reports, the Emla label, product information (PI), package insert and consumer medicine information (CMI) have been revised to stress adherence to the maximum recommended dose and application time.
Who is most at risk?
Children – especially those under 3 months – are at increased risk of serious adverse effects with overdose.
For circumcision in neonates and young infants (0–3 months), the package insert now specifies a maximum application time of 1 hour. The TGA is currently working with sponsors of the generic products to align their PIs, CMIs, labels and inserts with these changes.




 Kelly Abbott MPS[/caption]
Kelly Abbott MPS[/caption]


 Owner of Canberra's Capital Chemist Southlands Louise McLean MPS.[/caption]
Owner of Canberra's Capital Chemist Southlands Louise McLean MPS.[/caption]