From emergency contraception to migraines, advance supply can be appropriate. Here’s how to decide when it is – and isn’t.
It might sound like an odd request. Someone requesting a Pharmacist Only Medicine (S3) to have on hand for anticipated use in the future, rather than to respond to a current ailment or symptom. Is this allowed? And if so, when is it appropriate?
Is advance provision of S3s legal?
The answer is a (qualified) yes. While one of the primary reasons a medicine may be listed in Schedule 3 of the SUSMP,1 no Australian jurisdiction restricts advance provision of Pharmacist Only Medicines (see table).
But don’t we need to assess a patient’s therapeutic need?
Yes, the pharmacist must be satisfied there is a therapeutic need for the S3 medicine. This is mandated both in the Professional Practice Standards 2023, as well as (most) state and territory poisons regulations.
However, that therapeutic need doesn’t necessarily need to be for immediate treatment.
There will be circumstances where a therapeutic need for future use exists. For example, the APF2 treatment guidelines for emergency contraception, adrenaline and cold sores supports advance provision.
Similarly, there are cases – albeit less frequently – where advance provision is appropriate for treatment of conditions such as migraine, hives, allergic rhinitis or nasal congestion.
So when is advanced supply of S3 OK?
Quite often, but not always. Advance provision is most appropriate where a patient will need the medicine as time-sensitive treatment for a flare-up of a condition or expected recurrence of an ailment.
Advance provision is also more appropriate where a patient is unlikely to have reasonable access to a pharmacy, which is not limited to international travel.
Advance provision may not be appropriate where the patient may not be able to self-assess appropriate treatment for symptoms after counselling, where a therapeutic need does not exist, or if you believe there is a strong risk of diversion (although this is rare).
What other things do I need to consider?
There are a few, including:
- likelihood the medicine will expire prior to the patient using the medicines
- whether a patient’s other medicines or medical conditions (e.g. renal function) are likely to change and lead to drug-drug or drug-condition interactions.
These factors should be addressed in discussion with the patient when advance provision occurs.
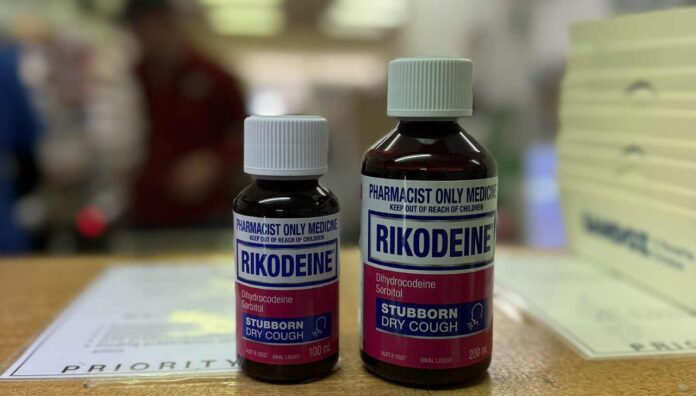
So what about the Rikodeine?
As an opioid analogue for treatment of dry cough, it will be pretty unlikely a patient will have a reasonable therapeutic need for advance provision of dihydrocodeine syrup.
However, each request should be considered on its own merits. There will be isolated cases where it is appropriate.
Table 1 – Legal requirements for pharmacists when prescribing Schedule 3 medicines
| ACT3 | No comparable criteria |
| NSW4 | · pharmacist gives the person an opportunity to seek advice as to the use of the substance
· quantity, or for a purpose must accord with the recognised therapeutic standard of what is appropriate in the circumstances |
| NT5 | · pharmacist must not intentionally supply to a person in a manner reckless to their circumstance |
| QLD6 | · pharmacist reasonably believes the patient has a therapeutic need |
| SA | No comparable criteria |
| TAS7 | · pharmacist forms the opinion use in the treatment of the patient is justified on consideration of the condition, disease or symptoms of the person |
| VIC8 | · for treatment of a patient under pharmacist’s care
· pharmacist has taken all reasonable steps to ensure a therapeutic need exists |
| WA9 | No comparable criteria |
Disclaimer: does not include requirements regarding additional controls for specific medicines, such as pseudoephedrine. Also does not include regulations (where permitted) for veterinary use, or circumstances such as supply to other health practitioners/first aid etc.
References
1. Australian Government Department of Health, Disability and Ageing. Therapeutic Goods Administration. SUSMP: Standard for Uniform Scheduling of Medicines and Poisons. 2023.
2. Pharmaceutical Society of Australia. Australian Pharmaceutical Handbook and Formulary 26th ed. 2024.
3. ACT Government. Medicines, Poisons and Therapeutic Goods Regulation 2008 | Subordinate laws
4. NSW Government. Section 18 Poisons and Therapeutic Goods Regulation 2008 – NSW Legislation
5. Northern Territory Government. Section 37(1) MEDICINES, POISONS AND THERAPEUTIC GOODS ACT 2012.
6. Queensland Government. Section 161 Medicines and Poisons (Medicines) Regulation 2021
7. Tasmanian Government. Regulation 58 Poisons Regulation 2018 Tasmanian Legislation Online
8. Victorian Government. Regulation 141 Drugs, Poisons and Controlled Substances Regulations 2017
9. Government of Western Australia. Medicines and Poisons Regulations 2016 – [00-r0-00].pdf



 Supplied by CSL Seqirus[/caption]
Supplied by CSL Seqirus[/caption]


 Tahnee Simpson[/caption]
Tahnee Simpson[/caption]
 Nicolette Ellis MPS[/caption]
Nicolette Ellis MPS[/caption]

 Ruth Nona[/caption]
Ruth Nona[/caption]