Case scenario
In a compounding pharmacy, Mrs Smith hands you a prescription for tacrolimus 0.02% eye drops for her dog, Biscuit. Biscuit suffers from severe dry eyes and the vet has prescribed tacrolimus 0.02% eye drops as additional relief on top of the lubricating eye drops Biscuit already uses. Biscuit trialled commercially available ciclosporin eye drops, but they did not help.
Introduction
Sterile compounding represents a vital aspect of pharmacy practice where sterility assurance procedures are paramount. This specialised field involves the preparation of sterile pharmaceuticals in a specifically designed facility, by specifically trained and competent personnel following specific standard operating procedures (SOPs) and aseptic processes. Similar sterility assurance procedures must be followed when manipulating or repackaging sterile commercial products.
Compounding must comply with the Pharmacy Board of Australia (PBA) Guidelines on Compounding of Medicines and should follow the Australian Pharmaceutical Formulary and Handbook (APF) compounding guidelines.
Learning objectivesAfter reading this article, pharmacists should be able to:
Competency standards (2016) addressed: 1.1, 1.4, 1.5, 2.2, 3.1, 3.4, 3.5 Accreditation number: CAP2406AMJT Accreditation expiry: 31/05/2027 |
Already read the CPD in the journal? Scroll to the bottom to SUBMIT ANSWERS.
The PBA guidelines and the new APF Compounding sterile medicines chapter highlight that pharmacists must comply with the principles contained in one of the following key standards when compounding sterile medicines1,2:
- Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S) – Guide to Good Manufacturing Practice for Medicinal Products and relevant annexes (PE009)
- PIC/S Guide to Good Practices for the Preparation of Medicinal Products in Healthcare Establishments and relevant annexes (PE010)
- United States Pharmacopeia (USP) —National Formulary: <797> Pharmaceutical compounding – sterile preparations (USP<797>).
The choice of standard depends on the facility’s objectives and regulatory requirements. Most sterile compounding facilities follow USP<797> or PIC/S PE010, while PIC/S PE009 is reserved for facilities seeking TGA licensing.
Quality and sterility assurance
The quality (including sterility) of an individual unit of a compounded sterile medicine cannot be guaranteed without testing the unit. However, this ‘end-product testing’ would destroy the unit.3
Pharmacists can compound medicines expected to be sterile and of the required quality by rigorously following a quality assurance system that incorporates relevant standards
and guidelines and the principles of Good Manufacturing Practice (GMP). These principles are based on the tenet that quality must be built into a product during all stages of the manufacturing process.4
Parametric release (also known as real time release testing (RTRT)) is a system of release that provides assurance that the final preparation is of the intended quality based on information collected during the compounding process (e.g. indicators of sterilisation) and on compliance with specific GMP requirements.5
Principles of GMP that apply to sterile compounding include1:
- using personnel with appropriate skills in sterile compounding practices and aseptic techniques
- following appropriate SOPs, including cleanroom procedures, aseptic and sterilisation processes, monitoring of cleanrooms and equipment for contamination
- using appropriate ingredients and formulae
- performing accurate and precise calculations and measurements
- labelling, packaging, storing and handling ingredients and compounded medicines in accordance with quality and safety requirements
- using and maintaining appropriate facilities and equipment
- routinely validating sterile compounding techniques and processes (e.g. aseptic process simulations, sterility testing samples of sterile medicines)
- confirming final compounded medicines comply with quality requirements before release
- maintaining appropriate documentation and records for all procedures, including maintaining a recall system
- using a self-audit program and applying appropriate corrective actions when needed.
Validation and risk assessment
Both PIC/S PE010 and APF underscore the critical importance of process validation and risk assessment in sterile compounding. Validation, as defined by PIC/S PE010, is: “The risk based, systematic, GMP compliant and documented evidence that a defined process actually leads reproducibly to the required results”.6
 Documented risk assessment is instrumental to a systematic approach to addressing potential failure points and mitigation strategies. While not all risks can be eliminated, implementing sufficient mitigation measures to reduce the risk score to an acceptable level is key.1,6
Documented risk assessment is instrumental to a systematic approach to addressing potential failure points and mitigation strategies. While not all risks can be eliminated, implementing sufficient mitigation measures to reduce the risk score to an acceptable level is key.1,6
A risk assessment tool can help to assess risk and determine necessary risk mitigation measures (e.g. APF Compounding decision support and risk assessment tool, Failure mode and effects analysis tool).1,6
Facilities and equipment
Dedicated, purpose-designed cleanrooms and clean air devices (e.g. laminar flow cabinets, pharmaceutical isolators) with controlled air quality are required for sterile compounding, to minimise the risk of contamination. They must comply with Australian standards and regulatory requirements and be suitable for the sterile medicines prepared and the expiry dates assigned.
The International Organization for Standardization (ISO) classifies air quality according to the airborne particle count. PIC/S guidelines classify cleanrooms and clean air devices into four grades (A, B, C, D), with Grade A being the cleanest. The grades correspond to certain ISO classes, depending on particle size and count at rest and in operation.3,7,8 See Table 1.
Personnel garb, and cleaning, maintenance and monitoring requirements, are commensurate with the grade. The cleaner the grade, the more stringent the requirements.3,7,8
Table 1 – Comparison of cleanroom environments

Aseptic processing (manipulating sterile ingredients or sterilising ingredients by filtration) must take place within a cabinet, isolator or equivalent device that maintains ISO Class 5 or cleaner (Grade A) air quality during aseptic processing. The device should be located in a cleanroom. In addition, cleanrooms and clean air devices used for the preparation of hazardous sterile medicines require containment systems appropriate for the level of risk to personnel and the environment.1,3,7
Two typical setups for preparing non-hazardous sterile medicines are7:
- Grade A pharmaceutical isolator within Grade C or D cleanroom
- Grade A laminar flow cabinet within Grade B cleanroom.
Cleanrooms must have dedicated heating, ventilation and air conditioning (HVAC) systems and air-filtering systems. Cabinets, isolators and equivalent devices must have their own high-efficiency particulate air (HEPA) or ultra-low particulate air (ULPA) filter. There must be air pressure differentials (pressure cascade) and air locks between each grade of cleanliness, to prevent migration of contaminated air into higher grade areas.3,7,8 The design of cleanrooms and clean air devices is complex and requires specialist advice.
Environmental monitoring
In the context of cleanroom contamination, particles are categorised as either ‘viable’ or ‘non-viable’. Viable particles include bacteria and fungi that can proliferate and potentially compromise the sterility of preparations. Non-viable particles encompass airborne impurities, like dust and skin flakes. Particulate contamination seems ever-present in cleanrooms, and unsurprisingly, mostly originates from the personnel.3,7,9
Viable particle monitoring detects culturable micro-organisms in the air, on surfaces and on personnel. Non-viable particle monitoring detects airborne particles.3 Equipment such as particle counters and air samplers are used together with settle and contact plates for routine environmental monitoring.3,7,8
An annual facility inspection and certification conducted by an independent National Association of Testing Authorities (NATA) accredited certifier is essential to verify compliance with relevant standards.
Cleaning and residue testing
It is essential that all materials entering the cleanroom are either sterile or disinfected using suitable disinfectants. Dedicated equipment should be employed for each room. For instance, if multiple separate cleanrooms are in use, distinct mops and buckets should be allocated to each room.3,7
All cleaning agents and methods should undergo validation to confirm
their efficacy. Any chemicals used must be compatible with the surface materials, for example, chlorine-based disinfectants are known to corrode metal surfaces in cleanrooms. In practice, compounding pharmacists often vary the types of disinfectants on a rotational basis in an attempt to avoid microbial resistance. Periodic use of sporicidal cleaning agents is also necessary, to destroy microbial spores.3,7 Suppliers can provide technical data on antimicrobial activity and the required disinfectant exposure time.
Finally, the risk of contamination or cross-contamination must be considered, especially when compounding hazardous medicines. Residue testing for common cytotoxic and hormone residues is available (i.e. TestSafe NSW) to monitor and validate cleaning processes.
Personnel
Personnel engaged in sterile compounding must undergo comprehensive training and skills validation. This encompasses a range of competencies, such as proper handwashing techniques, the donning of appropriate cleanroom garb and personal protective equipment (PPE), a fundamental understanding of GMP, adherence to health and safety protocols, skills in aseptic technique, pharmaceutical microbiology, and a functional knowledge of the preparations and services provided.
To guarantee the proficiency of personnel in maintaining adequate hand hygiene, all staff members should complete handwashing training and have their competency verified (e.g. using a commercial hand hygiene testing kit). Furthermore, validation of cleanroom gowning and aseptic techniques (also known as ‘operator media fills’), should occur routinely (e.g. every 6 months).
Personnel who handle or compound hazardous medicines must have periodic health monitoring (e.g. pathology tests) in accordance with the level of risk and relevant regulatory requirements.1,10,11
Clean room garb
Clean room garb (clothing) is designed to contain skin and particle shedding by personnel and prevent it from dispersing outwards. The higher the cleanroom grade, the more extensive the clean room garb that is needed.
For Grade D, general protective suits, gloves, overshoes, and hairnets are typically adequate. In higher-grade environments, personnel are typically required to wear separate cleanroom undergarments (e.g. scrubs), one-piece suits made from materials that do not
shed particles, hair and beard covers, cleanroom footwear or overshoes, fluid resistant masks, and sterile gloves. Street clothes must not be worn into changing rooms that lead to Grade C or cleaner areas, to maintain the integrity of the cleanroom environment.7
Formulation
Considering whether a medicine can be compounded is an important step in sterile compounding. It includes identifying a suitable formula and ensuring the availability of appropriate ingredients for the formulation. Additionally, it may be necessary to confirm that pyrogen loads of ingredients are within defined specifications. Pyrogens (e.g. bacterial endotoxins), if present in large amounts and injected, may lead to febrile reactions and have severe consequences.3,12 Ingredients intended for the preparation of injectable medicines should be pyrogen tested and a certificate of conformity provided by the supplier.
Pyrogens can also be introduced from devices and equipment that come in direct contact with the compounded medicine. All equipment used in sterile compounding should either be purchased as sterile and free of pyrogens, or undergo sterilisation and depyrogenation as necessary.
Sterilising
APF notes the following options for preparing a sterile medicine1:
Aseptic processing:
- Compound with sterile starting materials using aseptic manipulations under Grade A conditions – the final preparation should be sterile
- Sterilise by filtration – pass a non-sterile solution through a sterile filter with pore size of 0.22 micron or less. The solution to be filtered should be prepared under Grade C or better conditions and filtration performed using aseptic manipulation under Grade A conditions. Filtration should be followed by filter integrity testing.
Sterilise by terminal sterilisation:
- sterilise the final preparation in its container using a validated dry heat, steam (autoclave) or irradiation process.
Sterilisation by filtration is the simplest choice for smaller compounding facilities, however some preparations (e.g. suspensions, viscous solutions) cannot be sterilised by filtration. Preparation compatibility with the filtering material and the absorption of constituent onto the membrane must also be considered. Anhydrous preparations cannot be autoclaved, and some medicines decompose when exposed to high heat or irradiation. Sterilisation by heat or irradiation may also change physical properties of the preparation (e.g. particle size of suspensions). Therefore, validation of the selected sterilisation process is crucial to ensure the preparation can withstand the chosen method.
Different sterilisation processes can affect the preparation’s expiry date, as described in APF and USP<797>.
Assigning expiry
APF advises that expiry dates for compounded sterile medicines should be based on the guidance provided in the APF or the USP–NF <797>, unless the pharmacist can document evidence that an expiry date based on other reputable guidance will achieve at least an equivalent outcome.
APF expiry dates closely align with USP<797> ’beyond-use dates’ (BUDs). This date marks the final time a compounded medicine can be safely used. Once this date has passed, the sterile medicine must no longer be used.
An additional concept is the ’in-use expiry,’ particularly relevant to multi-dose injectable vials and multi-use eye drops. The in-use expiry represents the duration for which the medicine can be used after first opening.
The APF Compounding sterile medicines chapter provides useful expiry date tables, which categorise formulations as:
- sterile or non-sterile ingredients
- preserved or preservative-free
- aqueous or non-aqueous
- injectable or non-injectable
- specified or not specified in a reputable formulary.
The tables specify maximum permissible expiry dates and in-use expiries for each category, that vary with the method of sterilisation and the storage temperature. Pharmacies conducting their own stability research, with documented reputable evidence of physical, chemical and microbiological stability can apply their own determined expiry dates.1
Where third parties have conducted their own reputable stability-indicating studies for their formulas, it is possible to apply this expiry as long as the exact formula and method are followed with the same exact ingredients, which may include proprietary bases.1
Testing
While parametric release is the primary method for compounded medicine
release, routine periodic testing remains essential to verify that compounded medicines consistently meet specifications until expiry.3,7
For sterile medicines, testing encompasses chemical, physical, and sterility aspects, e.g.3,7,13:
- Potency testing – verifies that the active pharmaceutical ingredient (API) concentration remains within specified limits until expiry.
- Sterility testing – verifies that the compounded medicine is free
from micro-organisms. - Preservative effectiveness testing – verifies that any included preservatives will eliminate common micro-organisms upon exposure.
An independent NATA accredited facility should be engaged to conduct these tests.
Documentation
Given the specialised and detailed nature of sterile compounding, comprehensive documentation is crucial. Documentation should include (but not be limited to) SOPs, compounding records, calibration certificates, training records, and controlled temperature logs (fridge, room temperature and freezer). Documentation must be kept for the period specified in state or territory legislation. The storage location for these documents should be secure, yet readily accessible, even off-site, in case of audits or recalls. 1,2
Knowledge to practice
Dosage forms that must be sterile (for human or animal patients) include injections, eye preparations, irrigations, implants, nebuliser solutions, peritoneal dialysis solutions and preparations used on open wounds or in an injured ear. Microbial or other contamination of sterile medicines can have serious consequences for the patient. The compounding, manipulation or repackaging of sterile medicines requires specific training, competencies, standard operating procedures, facilities, equipment, quality and environmental controls, and documentation.
Conclusion
Sterile compounding, though complex, offers rewarding opportunities to provide patients with specialised compounding solutions. A compounding pharmacy can consistently deliver sterile medicines that are safe and appropriate for the patient by following the relevant
standards and guidelines.
Case scenario continued
During the risk assessment, you noted that there are no known commercially available tacrolimus eye drops. You also noted that APF lists tacrolimus as a medicine requiring additional special handling precautions. Given it is a hazardous and sterile medicine, a specialised sterile facility is essential to compound this eye drop safely. Consequently, you refer Mrs Smith to a compounding pharmacy which has the appropriate facilities.
This article is accredited for group 2 CPD credits. Click submit answers to complete the quiz and automatically record CPD against your record.
If you do get an enrolment error, please click here![]()
Key points
- Documented validations and risk assessments are instrumental in preparing sterile medicines that are safe and appropriate for the patient.
- All sterile medicines must be prepared in a dedicated, regularly tested and cleaned facility that is compliant with relevant standards and guidelines. This includes dilution, reconstitution or repackaging of commercial products.
- All personnel working in sterile compounding must have demonstrated and validated good aseptic technique, hand hygiene and garbing practices.
- Expiry dates should be assigned in accordance with APF or USP<797>.
References
- Sansom LN ed. Australian pharmaceutical formulary and handbook. 26th edn. Canberra: Pharmaceutical Society of Australia; 2024.
- Pharmacy Board of Australia. Guidelines on compounding of medicines. At: www.pharmacyboard.gov.au/Codes-Guidelines.aspx
- United States Pharmacopoeial Convention. USP-NF compounding compendium. 2023.
- Therapeutic Goods Administration. Good manufacturing practice – an overview. 2017. At: www.tga.gov.au/good-manufacturing-practice-overview
- European Medicines Agency. Guideline on Real Time Release Testing (formerly Guideline on Parametric Release). 2012. At: www.tga.gov.au/resources/resource/international-scientific-guidelines/international-scientific-guidelines-guideline-real-time-release-testing-formerly-guideline-parametric-release
- American Society for Quality. Failure mode and effects analysis (FMEA). At: https://asq.org/quality-resources/fmea
- Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S). Guide to Good Practices for the Preparation of Medicinal Products in Healthcare Establishments and relevant annexes (PE010).
- Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S). Guide to Good Manufacturing Practice for Medicinal Products and relevant annexes (PE009).
- Whyte W, Derks M. Airborne particle deposition in cleanrooms: relationship between deposition rate and airborne concentration. Clean Air and Containment Review. 2016;25:4–10.
- Victorian Pharmacy Authority. Victorian Pharmacy Authority Guidelines 2023. At: www.pharmacy.vic.gov.au/
- Pharmacy Regulation Authority SA. Guidelines for the operation of pharmacy premises by pharmacy services providers 2018. At: www.pharmacyauthority.sa.gov.au/
- Lund W, ed. The pharmaceutical codex. 12thedn. London: Pharmaceutical Press;1994.
- British Pharmacopoeia Commission. British Pharmacopoeia. London: BPC; 2023.
Our author
JOE TSAI BPharm(Hons), MPharmPract, BCGP has worked in a variety of pharmacy settings. Initially from New Zealand, Joe has worked in Melbourne since 2010 and started in his current role in a sterile compounding facility at Slade Pharmacy, Richmond in 2019, where he oversees quality and clinical trials.
Our reviewer
BRANKO RADOJKOVIC DiplPharm MClinPharm FSHP FANZCAP (Compound, Ophthal) AFHEA




 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.

 Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4
Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4  C – Comorbidity and risk factor management
C – Comorbidity and risk factor management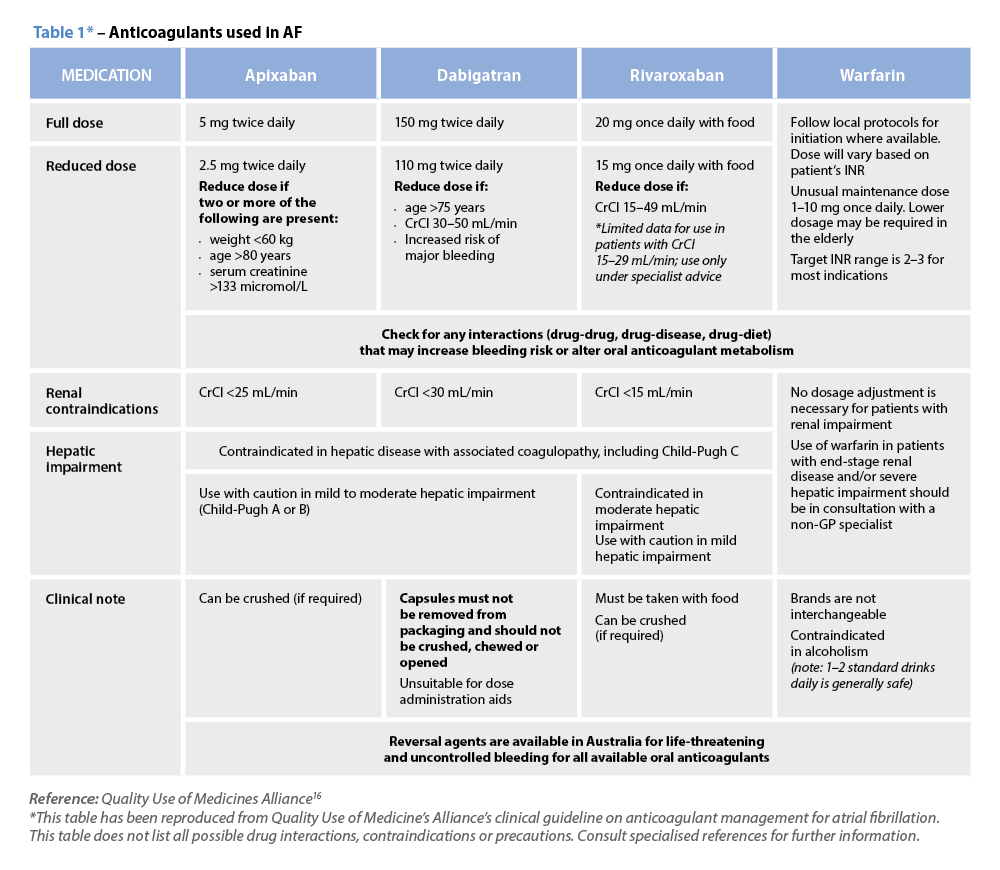 Warfarin
Warfarin






