Research from Curtin University published in 2017 found that pharmacists providing advice to lactating mothers who are seeking complementary medicines are finding the lack of safety data on the products a barrier to effective patient care.
Interviews were conducted with 30 community pharmacists from Western Australia, through which the participants’ education, confidence, strategies and continuing professional development (CPD) were assessed in light of evidence that women are likely to self-medicate and use non-prescription medicines while breastfeeding.1
‘I can’t be sure that what I recommend to them would be safe, so to be safe you would recommend less, and then probably the mum goes through a bit more suffering than she might need to, because we err on the side of safety,’ one participant said.
Previous research in Queensland found that 78% of pharmacists reported seeing women with infants on a daily basis.2 Community pharmacist accessibility and regular contact with breastfeeding mothers present ‘an invaluable opportunity for pharmacists and pharmacy staff to provide ongoing support to women during lactation’, especially in relation to the appropriate use of non-prescription medicines.1
A participant of the 2017 study expressed their uncertainty when faced with providing advice on using non-prescription medicines.
‘You really don’t have a clear decision on what is best for the patient as such. It would be better if we say no don’t use it or yes it’s safe use it. There isn’t enough data or there aren’t enough people in the test study to come to a proper conclusion,’ the participant said.
The researchers attributed participating pharmacists’ hesitance in recommending such medicines to the lack of available evidence-based safety data.
‘Whilst medication safety is within the field of expertise of pharmacists, the absence of evidence-based data was seen as a major challenge and barrier to enable pharmacists to confidently provide recommendations,’ the researchers wrote.
Participants also raised the issue of the lack of standardisation of herbal medicinal products.
‘I think because there [are] more trials and research done with prescribed and over-the-counter medicines, whereas with herbal medicines, especially if they are not standardised as well, that, and there’s not as much evidence, so that sort of reduces my confidence,’ one participant said.
‘I usually find that [in] the case with complementary medication, when I can’t find it [information] that’s when I would go on the side of caution and don’t recommend it and tell them there hasn’t been enough studies done about it,’ another participant said.
The researchers reported that all participants consistently mentioned they would not recommend the use of any medicines by breastfeeding women in the absence of evidence demonstrating their safety for the mothers and their infants.
‘In the absence of available evidence-based information, health professionals in many cases were required to consider the risk to baby versus benefit to mother,’3 the researchers wrote, referencing other studies done in the area.
In line with the PSA code of ethics and PSA position on complementary medicines, the researchers found literature to support raising the awareness and knowledge of pharmacists in regard to the safety of commonly used medicines in breastfeeding, in order to decrease the tendency to give conservative recommendations unnecessarily and to improve quality use of medicines during breastfeeding.4
‘Taking into consideration the prevalence of herbal medicines use amongst breastfeeding women in Western Australia and to meet their expectations, the perceived lack of knowledge and confidence of pharmacists, specifically in the area of complementary medicines use, should be addressed.’
The researchers concluded that training in this area was needed at both university and CPD level, and further studies into the safety of non-prescription medicines in breastfeeding were warranted.
The Australian Pharmaceutical Formulary and Handbook (APF) is a useful practice support tool that contains information on the safety of medicines in pregnancy and lactation. In particular, the Complementary medicines monographs, the Counselling Guides and the Provision of Pharmacist Only medicines guidance documents contain information on the use and safety of commonly used non-prescription medicines in pregnant and breastfeeding women.
Read the full article here (paywall)
References
- Sim TF, et al., The use of non-prescription medicines during lactation: A qualitative study of community pharmacists’ attitudes and perspectives, Research in Social and Administrative Pharmacy 2017;14(5)464-70.
- Hughes R, Maher J, Baillie E, Shelton D. Nutrition and physical activity guidance for women in the pre- and post-natal period: a continuing education needs assessment in primary health care. Aust J Prim health. 2011;17(2):135-141.
- Amir LH, Pirotta MV. Medicines for breastfeeding women: a postal survey of general practitioners in Victoria. Med J Aust. 2009;191(2):126.
- de Ponti M, Stewart K, Amir LH, Hussainy SY. Medicine use and safety while breastfeeding: investigating the perspectives of community pharmacists in Australia. Aust J Prim Health. 2013;21(1):46-57.



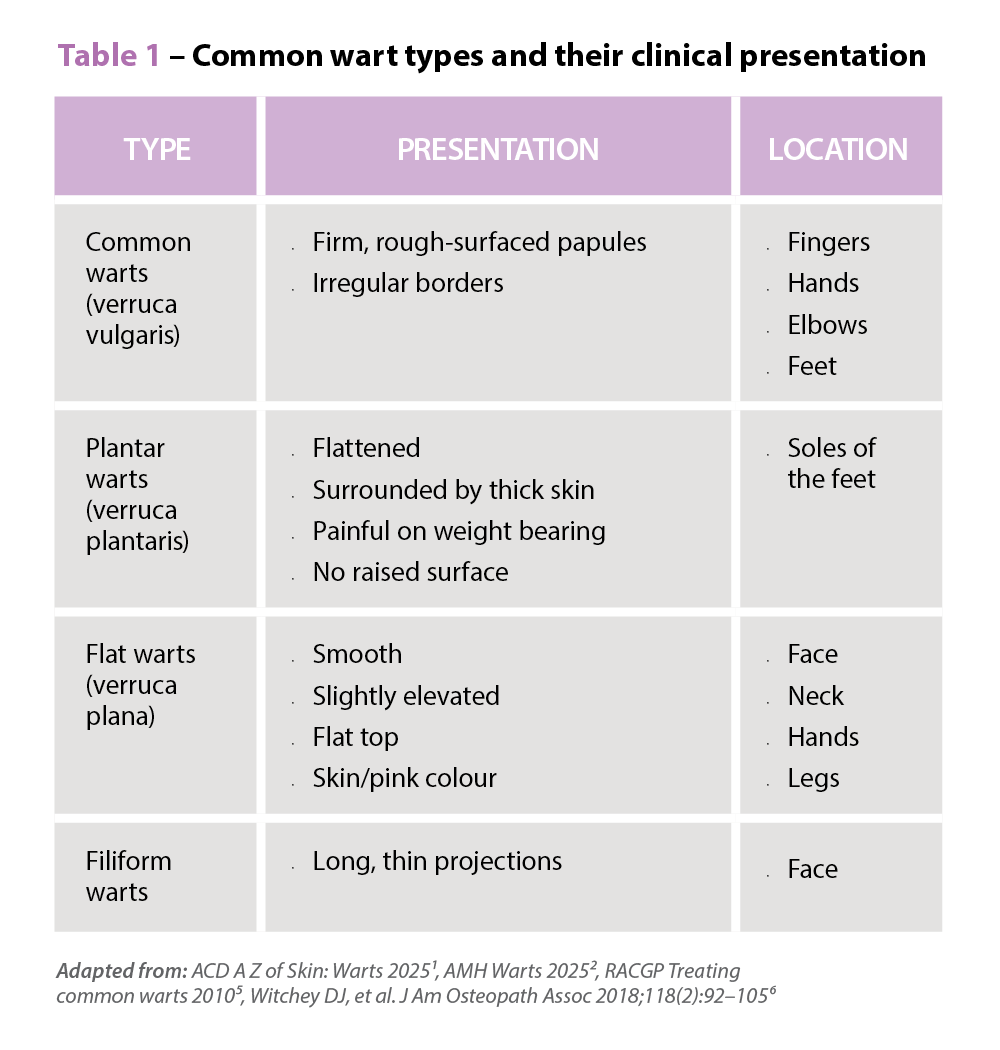 Symptoms
Symptoms