Practical steps for pharmacist intervention against respiratory syncytial virus (RSV).
Until recently, most consumers had never heard of respiratory syncytial virus (RSV). Suddenly its seemingly everywhere, with rapid antigen tests for the virus and GPs screening for RSV alongside COVID-19 and influenza.
Reports of the highly infectious virus have surged across Australia, with 128,013 cases registered last year. In NSW alone, reported cases rose from 5,965 in 2022 to 46,531 in 2023 (at time of publication).1
There are also vaccines under development. In January 2024 the Therapeutic Goods Administration (TGA) approved the first respiratory syncytial
virus vaccine – Arexvy.2
On 29 February the Australian Technical Advisory Group on Immunisation (ATAGI) issued guidance, recommending the vaccine for all people ≥75 years age and some people from 60 years of age (see Figure 1).
Often associated with young children the burden of severe disease and death actually rests with older persons.
In severe cases, infection can spread lower causing pneumonia or bronchiolitis. But emerging evidence points to a risk of long-term impacts for older Australians who contract RSV.
The virus, spread through aerosol droplets formed from coughs or sneezes, can be particularly dangerous for people who are frail or suffering from chronic health conditions.
So where did RSV originate? And should patients – and pharmacists – be worried?
Ask the expert
Debbie Rigby FPS*, Advanced Practice Pharmacist
 What is RSV?
What is RSV?
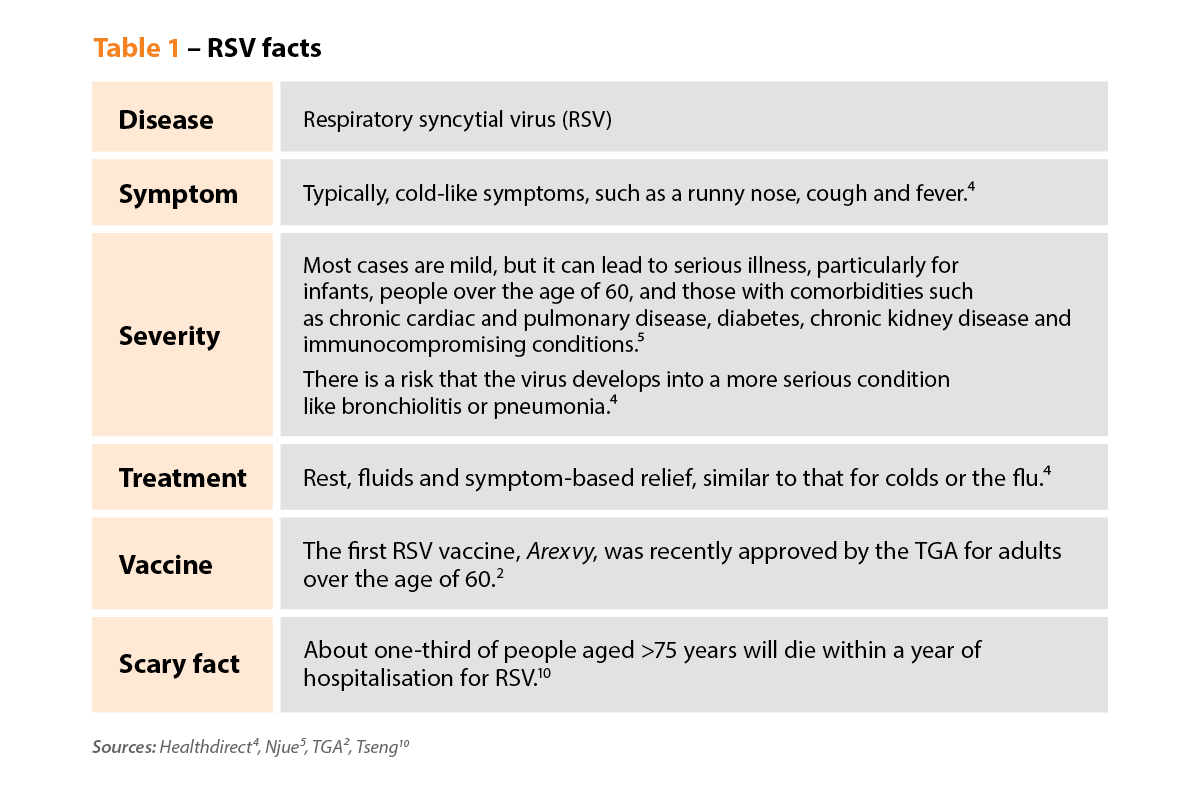
RSV, or respiratory syncytial virus, is a common respiratory disease that causes cold-like symptoms. People often think of children when they think of RSV because it’s very common in young kids. Virtually all children are infected before the age of two.
RSV can cause serious illness in very young children, and they typically present with bronchiolitis. But in recent years there’s been a greater awareness of RSV infection in adults, particularly those over the age of 60.
Where did it come from?
RSV has been around for a long time, but it’s been under-recognised. The virus only became a notifiable disease in 2021, so there are no good statistics on its prevalence, especially in older age groups.
We do know that RSV is a very contagious virus. Individuals infected are likely to each infect three other people. And any natural immunity to RSV is very short-lived, so repeated infections throughout life are typical.
Most adults aged over 60 have probably had RSV 6–10 times. But their symptoms may have been mild and may have been interpreted as a common cold or influenza.
How does it present?
People with RSV tend to present with a runny nose, coughing and sneezing – cold-like symptoms, rather than flu-like symptoms.
Taking a good history and asking patients about their symptoms is important. But it can be challenging to differentiate from the common cold.
Can you test for RSV?
Triple combination rapid antigen tests (RATs) incorporating influenza A and B, RSV and COVID-19 are available over the counter at community pharmacies. There are also PCR tests now that analyse a multiplex respiratory panel. So, pathology laboratories test for RSV, influenza A and B, COVID-19, adenovirus and others – a range of respiratory diseases.
How is it treated?
There’s usually no specific medicine for RSV, only symptom-based treatment similar to that for colds or the flu. The exception is monoclonal antibodies, such as nirsevimab (Beyfortus), which can be given to severely immunocompromised babies and young children.3 Abrysvo, a Pfizer RSV vaccine has been approved in the United States for use by pregnant women but has not yet been approved in Australia.
Who is most at risk?
Older adults – aged 60 and over – are at high risk of severe RSV infection. The older you are, the more serious RSV infection is likely to be. And if you have comorbidities, the risks are even higher. One quarter of the Australian population is at risk of severe infection, which can result in hospitalisation – and even death.
The biggest risk is for older people with comorbidities like asthma, diabetes, chronic obstructive pulmonary disease (COPD), heart failure, chronic kidney disease and coronary artery disease.
That’s an important message that pharmacists need to understand and convey to older adults.
People of any age who are immunocompromised are also at greater risk of getting RSV and of having a more severe infection requiring hospitalisation.
 Are there any long-term impacts?
Are there any long-term impacts?
There’s a lot of emerging evidence about the long-term effects of an RSV infection in older people.6–8
The statistics are somewhat startling. About a quarter of people aged over 60 who are admitted to hospital with RSV will require home care when discharged. And about a quarter will be readmitted to hospital within 3 months.9
I think the really alarming number is that about a third of people aged 75 years and older will die within 1 year of admission to hospital with RSV.10 So, many, many people won’t get back to their normal functional level.
It can really impact on people’s quality of life, their emotional, physical and cognitive functioning, and their sleep. It’s a little bit like the long-term effects we’re seeing after COVID-19 infection.
Is there a vaccine?
Yes! Three RSV vaccines have been going through clinical trials. One of them one, the GSK vaccine [Arexvy], has just been approved by the TGA for adults aged over 60.
It’s highly effective, leading to an 83% reduction in the incidence of lower respiratory tract symptoms and a 94% reduction in severe RSV infection.11
Presently, the vaccine is only available on private prescription. But I expect it will be added to the National Immunisation Program in time.
For whom is the vaccine recommended?
The vaccine, indicated for ‘active immunisation of individuals 60 years and older for the prevention of lower respiratory tract disease’ caused by RSV,12 is particularly important for older Australians with those comorbidities – asthma, diabetes, COPD, heart failure, chronic kidney disease and coronary artery disease.
There’s also good evidence of clinical outcomes for people who are frail. I’d recommend it in these groups.
ATAGI has recommended vaccination for all people aged 75 years and above, people 60–74 years of age with medical conditions, which inrease risk of severe disease due to RSV, and Aboriginal and Torres Strait Islander people over the age of 60 years (see Figure 1).
It does need to be a shared decision. Pharmacists can help patients understand their individual risk, and the impact on their quality of life, social and leisure activities.
Is there an RSV season?
Typically, in our temperate and colder climates RSV has a late autumn to winter season, similar to influenza.
In subtropical areas like Brisbane, it can be earlier because of the warmer weather. And in Northern Australia, it can be in the wet season. But anytime is a good time to get vaccinated against RSV. According to the vaccine product information, a need for revaccination has not been established.13
 Of what else should pharmacists be aware?
Of what else should pharmacists be aware?
There is evidence that RSV infections can exacerbate existing medical conditions.
It makes people much more likely to be hospitalised with heart failure, asthma or COPD. Among patients with COPD, 80% experienced an exacerbation during a RSV-associated hospitalisation.10
It’s important for pharmacists to support patients, as we always do, to better manage those comorbidities – actually taking their medicines or using their preventers in the case of asthma and COPD.
*Debbie Rigby FPS is a member of the GSK and Moderna advisory board for RSV.
** Opinions expressed are those of the expert author, and may not represent the views of PSA.
References
1. Department of Health. National Communicable Disease Surveillance Dashboard. Canberra: DoH. 2024. At: https://nindss.health.gov.au/pbi-dashboard
2. Australian Government Department of Health and Aged Care. Therapeutic Goods Administration. Arexvy. 2024. At: www.tga.gov.au/resources/auspmd/arexvy
3. Australian Government Department of Health and Aged Care. Therapeutic Goods Administration. Beyfortus. 2023. At: www.tga.gov.au/resources/auspmd/beyfortus#:~:text=Beyfortus%20(nirsevimab)%20was%20approved%20for,entering%20their%20first%20RSV%20season.
4. HealthDirect. Respiratory syncytial virus (RSV) [Internet]. HealthDirect Australia; 2024. At: www.healthdirect.gov.au/respiratory-syncytial-virus-rsv
5. Njue A, Nuabor W, Lyall M, et al. Systematic literature review of risk factors for poor outcomes among adults with respiratory syncytial virus infection in high-income countries. Open Forum Infect Dis 2023;10(11):ofad513.
6.Centres for Disease Control and Prevention. Respiratory Syncytial Virus Infection (RSV), RSV in older adults and adults with chronic medical conditions. 2024. At: www.cdc.gov/rsv/high-risk/older-adults.html#:~:text=RSV%20can%20sometimes%20also%20lead,and%20oxygen%20through%20the%20body
7. Juhn YJ, Wi CI, Takahashi PY, et al. Incidence of respiratory syncytial virus infection in older adults before and during the COVID-19 pandemic. JAMA Netw Open 2023;6(1):e2250634. At: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2800696
8. Mayo Clinic. RSV and older adults: who’s at risk? 2023. At: https://mcpress.mayoclinic.org/rsv/rsv-and-older-adults-whos-at-risk/
9. Falsey AR, Walsh EE, House S, et al. Risk factors and medical resource utilization of respiratory syncytial virus, human metapneumovirus, and influenza-related hospitalizations in adults – a global study during the 2017–2019 epidemic seasons (Hospitalized Acute Respiratory Tract Infection [HARTI] study). Open Forum Infect Dis 2021;8(11):ofab491. At: www.ncbi.nlm.nih.gov/pmc/articles/PMC9088513/
10. Tseng HF, Sy LS, Ackerson B, et al. Severe morbidity and short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis 2020;222(8):1298–1310. At: https://academic.oup.com/jid/article/222/8/1298/5863549
11. Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein Vaccine in Older Adults. N Engl J Med 2023;388:595–608. At: www.nejm.org/doi/full/10.1056/NEJMoa2209604
12. Australian Technical Advisory Group on Immunisation (ATAGI). Clinical Advice. Version 1.0. March 2024.
Statement on the clinical use of Arexvy (RSV PRE-F3) vaccine for prevention of respiratory syncytial virus (RSV) disease in older adults in Australia. At: www.health.gov.au/sites/default/files/2024-02/atagi-statement-on-the-clinical-use-of-arexvy-rsv-pre-f3-vaccine-for-rsv.pdf
13. Therapeutic Goods Administration. Product Information. 2024. At: www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2024-PI-01071-1&d=20240215172310101
14. NSW Government NSW Health. Pharmacist initiation and administration of vaccines. 2024. At: www.health.nsw.gov.au/immunisation/Pages/pharmacist-vaccination-expansion.aspx



 Kelly Abbott MPS[/caption]
Kelly Abbott MPS[/caption]


 Owner of Canberra's Capital Chemist Southlands Louise McLean MPS.[/caption]
Owner of Canberra's Capital Chemist Southlands Louise McLean MPS.[/caption]

 Supplied by CSL Seqirus[/caption]
Supplied by CSL Seqirus[/caption]