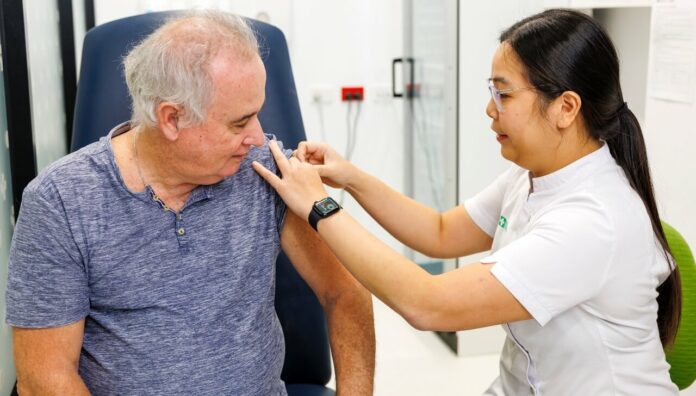
Since 1 November 2023, almost 5 million Australians at risk of severe complications from shingles have had access to the SHINGRIX vaccine under the National Immunisation Program (NIP).
Herpes zoster (HZ), a reactivation of the varicella-zoster virus in patients who have had chicken pox previously, has a significant disease burden. Of all vaccine preventable diseases, HZ and its complications accounted for 7% of the overall burden in 2015.
One of the most common complications from the virus is post-herpetic neuralgia (PHN), particularly among older and immunocompromised patients. Up to 5–30% of patients with HZ go on to develop PHN, with risks of this complication increasing with age.
PHN, a type of nerve pain described as having a burning sensation that can persist long after acute HZ symptoms resolve, can be debilitating.
One in three Australians will get shingles in their lifetime. Vaccination can reduce this likelihood.
What’s changed and why?
SHINGRIX replaced Zostavax on the NIP following advice from the Pharmaceutical Benefits Advisory Committee and the Australian Technical Advisory Group on Immunisation. SHINGRIX is administered as two doses, 2–6 months apart, at a current private market cost of approximately $560.
The age limit for NIP shingles vaccination eligibility is now 65 years and older, down from 70 years.
Patients eligible to receive SHINGRIX under the NIP include:
- those aged 65 years and older
- Aboriginal and Torres Strait Islander people aged 50 years and older
- immunocompromised people (with certain medical conditions) aged 18 years and older.
There are no studies directly comparing the efficacy of Zostavax (a live attenuated vaccine) and SHINGRIX (an adjuvanted recombinant virus subunit vaccine). However, when comparing clinical trial data the adjuvant recombinant subunit vaccine demonstrated higher vaccine efficacy against placebo than the live attenuated vaccine against placebo.

‘SHINGRIX also provides longer lasting protection than Zostavax, with a recent study showing immunity remained high for at least 7 years after vaccination with SHINGRIX,’ said PSA Policy Pharmacist Karen Castle MPS.
Which immunocompromised patients are eligible?
As a live attenuated vaccine, Zostavax is contraindicated for some at-risk patients, who now benefit from the NIP reform, said Ms Castle.
‘Immunocompromised patients 18 years and over can now access the vaccination via the NIP, whereas they couldn’t previously,’ she said.
Immunocompromised adults with the following medical conditions are eligible for an NIP-funded SHINGRIX vaccine:
- haemopoietic stem cell transplant
- solid organ transplant
- haematological malignancy
- advanced or untreated HIV.
Can pharmacists administer the vaccine?
The herpes zoster vaccines pharmacists can administer by jurisdiction in Australia include:
- New South Wales (SHINGRIX brand only)
- ACT (SHINGRIX brand only)
- Tasmania (SHINGRIX brand only)
- South Australia (any brand)
- Victoria (any brand)
- Western Australia (any brand)
- Northern Territory (any brand)
- Queensland (any brand).
Information about age restrictions and other conditions specific to the vaccine is available via PSA’s Pharmacist administered vaccinations microsite. Pharmacists must also follow the recommendations in the Australian Immunisation Handbook when selecting a herpes zoster vaccine.
Funding of $18.85 to administer all vaccines under the NIP occurred from 1 January 2024, including SHINGRIX. While vaccine costs are covered under the NIP, administration fees may apply.
What do patients need to know?
Pharmacists should use the NIP update as an opportunity to educate patients about the dangers of HZ, and that they may now be eligible to receive a free SHINGRIX vaccine.
‘Pharmacists can check their patients herpes zoster vaccination status on the Australian Immunisation Register,’ said Ms Castle.
‘If you do identify someone who is eligible for a herpes zoster vaccine, you should let them know the SHINGRIX vaccine is now free and it is more effective than Zostavax.’
Pharmacists should advise patients who have previously received the Zostavax vaccine that they can also receive SHINGRIX to increase their protection against herpes zoster, as Zostavax protection decreases over time.
However, patients should wait at least 12 months between receiving Zostavax and SHINGRIX, and will still need to comply with the two-dose schedule. The NIP specifies the waiting period between subsidised Zostavax and SHINGRIX doses.




 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.

 Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4
Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4  C – Comorbidity and risk factor management
C – Comorbidity and risk factor management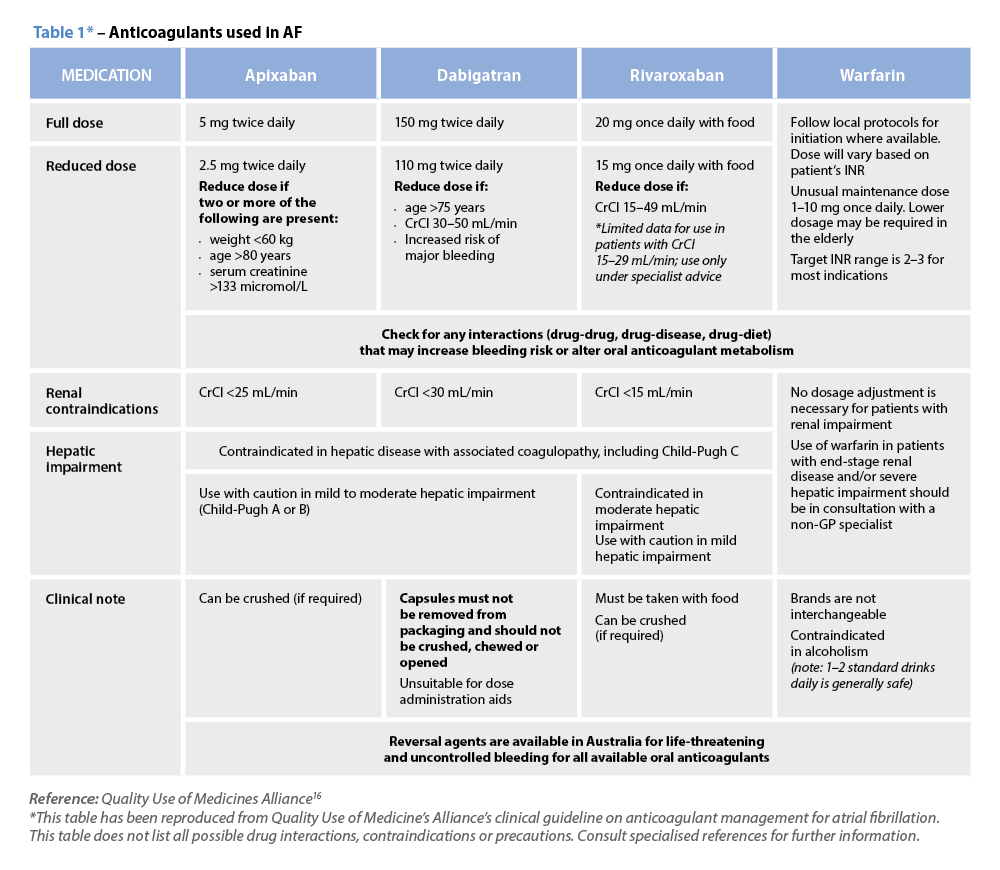 Warfarin
Warfarin