Medication errors and poisonings are leading to a staggering number of avoidable hospitalisations among children and adolescents. Find out which medicines pose the biggest risks and how pharmacists can help turn the tide.
An alarming number of Australian children and adolescents are impacted by medicine-related harm. Every day, around 93 children present to emergency departments due to a medicine-related problem – half of which are preventable.
And adverse events due to medicines are all too commonplace, with an estimated 120,000 children aged 0–14 years affected in the last 6 months.
Along with the emotional and physical toll on children and their families, medicine-related harm also carries a significant financial burden to the tune of $130 million per annum.
These alarming statistics, and more, were highlighted in PSA’s Medicine safety: child and adolescent care report, released on Friday by PSA National President Associate Professor Fei Sim and Ged Kearney, Assistant Minister for Health and Aged Care Assistant Minister for Indigenous Health.

‘Our health system is failing children and adolescents,’ said A/Prof Sim. ‘As a health community, we must commit to doing better, but we also need to be given the resources and tools to do better.’
Here’s a look at the key findings and the tangible solutions pharmacists can employ to make a difference.
Steep increase in self-poisonings among teens
Poisoning remains a major risk for children and adolescents, leading to around 8 hospitalisations daily.

Younger children are particularly susceptible to poisoning, often due to accidental ingestion or dosing errors. In children under 5 years of age, poisoning admissions increased from 12% in 2013–14 to 16% in 2022–23, with medicines affecting the cardiovascular system the most common cause.
While concerning, the number of hospitalisations among children aged 5–9 remains relatively low. But poisonings – both accidental and intentional – are increasingly prevalent in adolescents.
Paracetamol and antidepressants are the leading causes of poisoning-related hospital admissions, particularly among girls aged 10–14 years and adolescent females aged 15–19 years. In fact, 68% of all pharmaceutical poisoning admissions in 2022–23 were in adolescents aged 15–19 years.
Beyond hospital data, the New South Wales Poisons Information Centre (PIC) receives thousands of calls each year; among adolescents, calls about antidepressant exposures, including fluoxetine and sertraline, are increasingly prominent.
Off-label medicine use fuelling adverse reactions
Many medicines prescribed for children have not been specifically tested in paediatric populations, leading to widespread off-label use.
An estimated 12–45% of medicines used in children are off-label, increasing the risk of adverse reactions. And while medicine-medicine interactions are understudied in Australian children, international evidence suggests that exposure to major drug interactions in paediatric patients may be higher than anticipated.
With diagnoses of autism and ADHD on the rise – conditions which are linked to sleep disturbances – many parents are administering melatonin off-label to children, often obtaining it online and perceiving it as a ‘natural product’ rather than a medicine.
But the NSW PIC has received a growing number of melatonin exposure-related calls, with nearly 850 in 2022 alone.
Poisonings linked to clonidine, prescribed off-label for ADHD, are also frequently reported. Due to its tablet formulation, clonidine has been associated with dosing errors, particularly among young children – who may accidentally take a whole tablet instead of the prescribed half or quarter of a tablet.
Treating mental health conditions comes with risks
The significant uptick of mental ill health in today’s youth has led to a massive increase in the use of medicines for mental health conditions in children, including antidepressants and ADHD medications.
While these medicines can be beneficial, they are also associated with risks, including cardiometabolic side effects from antipsychotics and dependency risks from stimulants. A key issue is the lack of health literacy among parents and caregivers, leading to medication errors, inappropriate dosing, or failure to recognise adverse effects.
Pharmacists critical to harm prevention
The report’s release coincides with new restrictions on paracetamol pack sizes, which came into effect on Saturday 1 February, to reduce risks of intentional overdose. Pharmacy medicine packs are now limited to 50 tablets in most states, with larger packs upscheduled to Pharmacist Only medicines.
‘Paracetamol and antidepressants are the leading causes of poisoning-related hospital admissions, particularly among girls aged 10–14 years.’
‘By involving pharmacists in the supply of larger paracetamol pack sizes, we strike the right balance between access and safety, giving pharmacists and pharmacy assistants the opportunity to help patients manage their pain effectively while reducing the risk of misuse and harm,’ said A/Prof Sim.
The report suggests that mandating an indication on prescriptions could also ensure pharmacists verify the appropriateness of each medication. For example, when a child receives an antibiotic, pharmacists can confirm it’s suitable for the specific infection and the patient’s age before dispensing.
Accurate dosing is another key priority area. Given children’s dosage often depends on weight and age, pharmacists should manually check every paediatric dose rather than relying solely on automated systems. For example, if a 15 kg child is prescribed amoxicillin, pharmacists should calculate an appropriate mg/kg dose and contact the prescriber if it exceeds recommended limits.
From a systems perspective, designing a national medicine safety monitoring system – akin to Canada’s AIMS (Assurance and Improvement in Medication Safety) – will allow pharmacists to log and analyse medication errors in real time. This data-driven approach helps identify trends in paediatric medicine use, preventing repeated mistakes.
Removing legal barriers so pharmacists can modify medication formulations, such as creating a liquid formulation of clonidine for a child who can’t swallow pills, can also reduce errors and improve adherence.
But beyond clinical responsibilities, pharmacists should educate parents, teachers, and caregivers on medicine safety. This includes guidance on correct administration techniques, such as using a syringe for accurate measurement, and safe storage practices to prevent accidental ingestion.
‘Pharmacists are critical to ensure the safe use of medicines and must be supported to do so,’ said A/Prof Sim.
‘That means adequately staffing children’s hospital wards with the expertise of pharmacists, investing in systems that capture the data needed for evidence-based policy, and improving the quality use of medicines whenever medicines are used.
‘It takes all of us, across all areas of practice and indeed across all health professions, to make a difference to the children and adolescents who rely on our care.’
Access PSA’s full Medicine safety: child and adolescent care report here.




 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.

 Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4
Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4  C – Comorbidity and risk factor management
C – Comorbidity and risk factor management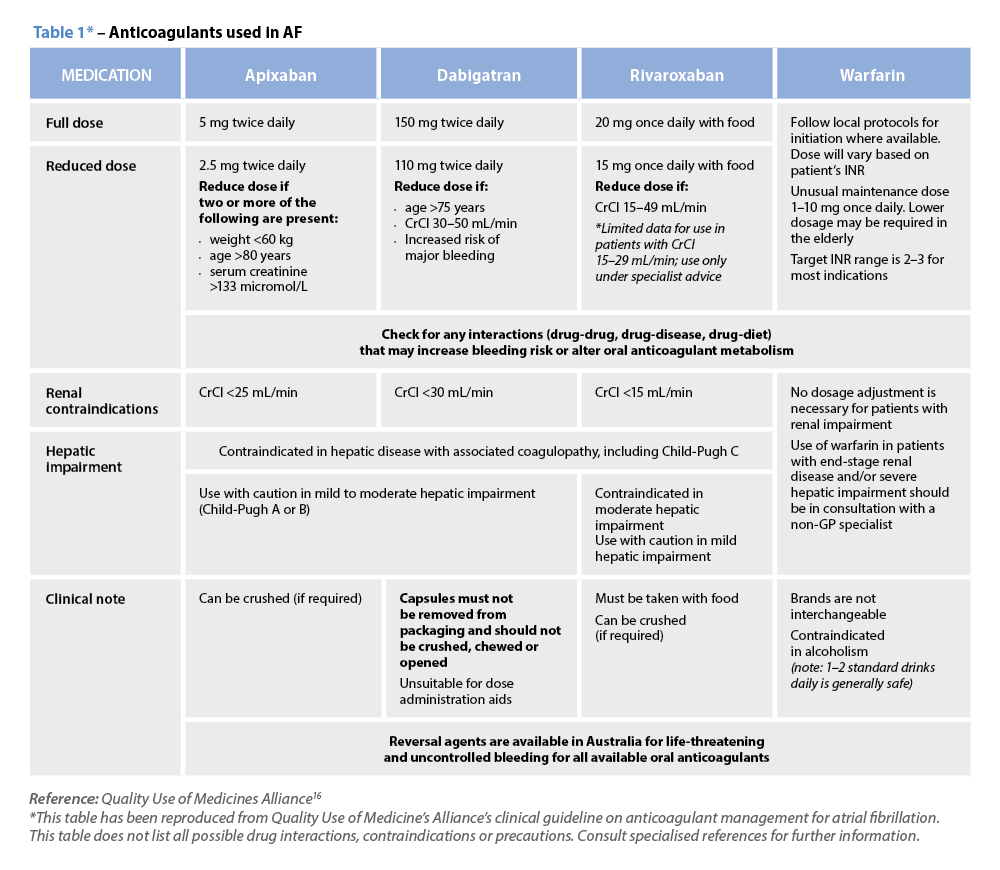 Warfarin
Warfarin




