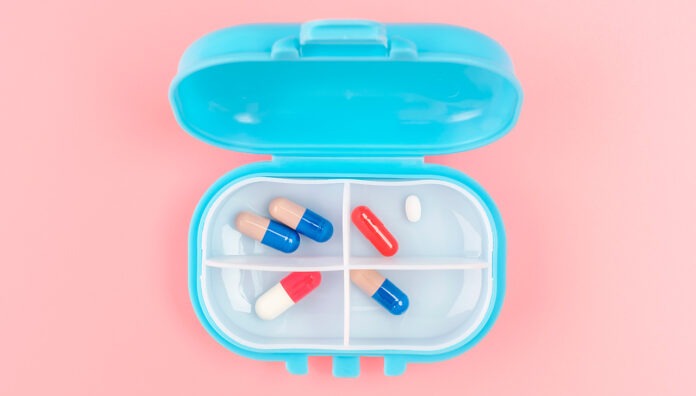
Patients often find creative ways to remember to take their medicines. These might include alarms and reminder apps or placing medicines in visible locations like kitchen benches or
bedside tables. However, some patients remove medicines from their original packaging to make their medication routine more convenient.
They might transfer them into pill organisers, plastic bags or leave them loose in pockets or
handbags. While these strategies may help with adherence, they can also compromise
medicine stability and effectiveness.1
Challenges of repackaging medicines
While manufacturers test the stability of medicines in original packaging, this assurance of stability over the shelf life is lost once medicines are removed from the original packaging.2,3
A national study of community pharmacists found that 88% had observed visible changes
in repackaged medicines, including discolouration, softening and enteric coat rupture. These changes were most frequently reported in humid and hot climates.1
In these high-risk climates, sodium valproate, telmisartan, and aspirin were found to be particularly susceptible to physical instability.1
Patient storage practices: a critical factor
Despite pharmacist recommendations, patients may unknowingly compromise their medicines’ safety by exposing them to heat, moisture or light, leading to degradation and reduced effectiveness. Common storage locations (e.g. bathroom cabinet, car) can contribute to degradation.
For instance, enteric-coated tablets, designed to resist stomach acid, were found to rupture prematurely when exposed to moisture. Softening of tablets and capsules was also frequently noted, leading to crumbling or loss of integrity. These changes can compromise drug efficacy and patient safety, particularly for medicines with a narrow therapeutic index.4
Best practices for medicine storage at home
To maintain medicine stability and effectiveness, patients should follow key storage principles:
- Control temperature: Medicines prone to heat degradation, such as telmisartan, should be stored in a cool, dry place. Refrigeration may be necessary for some medicines, as advised by a pharmacist.5
- Protect from moisture: Medicines should not be stored in humid environments like bathrooms.6
- Protect from light: Some medicines degrade when exposed to light and specific storage instructions on the packaging should be followed.
- Recognise degradation: Any physical changes to medicines, (e.g. colour, texture, odour) should be reported to a pharmacist immediately.
The pharmacist’s role in providing medicines information
Pharmacists play a crucial role in ensuring patients understand the importance of proper medicine storage. When providing medicines information to patients on initiation or continuation of a medicine, pharmacists should routinely ask them how they store their medicines at home and provide tailored recommendations based on their responses.
Key points to include:
- ask where the patient currently stores medicines and address potential risks
- advise patients to keep medicines in their original packaging whenever possible
- highlight medicines that are particularly sensitive to heat, moisture or light
- encourage patients to report any changes in their medicines’ appearance or effectiveness.
The APF Good dispensing practice chapter provides information on expiry dates and storage conditions for repackaged medicines.
References
- Robertson SG, Bradshaw A, Glass BD. To repack or not to repack. Aust J Pharm 2022. At: https://ajp.com.au/in-depth/longer-read/to-repack-or-not-to-repack/
- Pharmacy Board of Australia. Guidelines on dose administration aids and staged supply of dispensed medicines. 2015. At: www.pharmacyboard.gov.au
- Pharmaceutical Society of Australia. Guidelines for pharmacists providing dose administration aid services. 2017. At: www.ppaonline.com.au/wp-content/uploads/2019/01/PSA-Dose-Admin-Aid-Guidelines.pdf
- Redmayne N, Robertson S, Kockler J, et al. Repackaged sodium valproate tablets – – Meeting quality and adherence to ensure seizure control. Seizure 2015;31:108–11.
- Robertson SG, Glass BD. Stability of repackaged dabigatran etexilate capsules in dose administration aids. Eur J Hosp Pharm 2018;25:e93–7.
- Donyai P. Quality of medicines stored together in multi‐compartment compliance aids. J Clin Pharm Ther 2010;35(5):533–43.



 Genevieve Adamo MPS (Image: Steve Christo Photography)[/caption]
Genevieve Adamo MPS (Image: Steve Christo Photography)[/caption]



 Deborah Williams at the Chemist Warehouse Australian Open pop-up pharmacy[/caption]
Deborah Williams at the Chemist Warehouse Australian Open pop-up pharmacy[/caption]