Following several incident reports, the Therapeutic Goods Administration (TGA) has issued new preoperative precautions linked to these popular therapies.
Last week, the TGA issued an alert over use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RAs) before procedures involving general anaesthesia or deep sedation.
This warning extends to all medicines in these classes approved for diabetes and/or chronic weight management, including:
- semaglutide (Ozempic, Wegovy)
- dulaglutide (Trulicity)
- liraglutide (Victoza, Saxenda)
- tirzepatide (Mounjaro).
What are the risks?
GLP-1 RAs and GIP/GLP-1 RAs are known to delay gastric emptying. So despite standard fasting periods ahead of surgeries or procedures, patients may have residual stomach contents that increase the risk of aspiration under general anaesthesia or deep sedation.
Last month, the TGA delved into the Database of Adverse Event Notification to track incidents linked to GLP-1 and dual GIP/GLP-1 receptor agonists – uncovering 11 reported adverse events.
These included:
- semaglutide: seven aspiration episodes and one aspiration-related pneumonia
- liraglutide: one aspiration and one pneumonia aspiration
- dulaglutide: one pneumonia aspiration case.
What advice should pharmacists provide?
Product Information for these medicines has been updated to include the following warning:
4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE:
Aspiration in association with general anaesthesia or deep sedation
Cases of pulmonary aspiration have been reported in patients receiving GLP-1 RAs undergoing general anaesthesia (GA) or deep sedation despite reported adherence to preoperative fasting recommendations. Therefore, the increased risk of residual gastric content because of delayed gastric emptying should be considered prior to performing procedures with GA or deep sedation.
It’s integral that pharmacists adjust their counselling advice accordingly, said Pooja Jadeja MPS from PSA’s Pharmacist to Pharmacist Advice Line.
‘Now that the TGA has issued this warning, pharmacists should mention it every first dispensing of these medicines,’ she said.
‘If the patient has used it before but is new to the pharmacy, pharmacists should also mention this information – just like they would for any other medicine that shouldn’t be taken prior to surgery or should be taken with caution in the case of a dental procedure.’
Patients should be encouraged to mention their GLP-1 medicine to any healthcare professional including their GP, dentist, or specialist, as this could affect how they are managed before any sedation or surgery, said Bill Wallace MPS, PSA Pharmacist – Professional Support Adviser.
‘Some people may be hesitant to mention they’re using a GLP-1 medicine for weight management, so it’s important they know it should always be discussed – especially before procedures,’ he said.





 Team PSA 2026: Caroline Diamantis FPS, Prof Mark Naunton MPS and Bridget Totterman MPS[/caption]
Team PSA 2026: Caroline Diamantis FPS, Prof Mark Naunton MPS and Bridget Totterman MPS[/caption]
 A/Prof Fei Sim and Prof Mark Naunton[/caption]
A/Prof Fei Sim and Prof Mark Naunton[/caption]
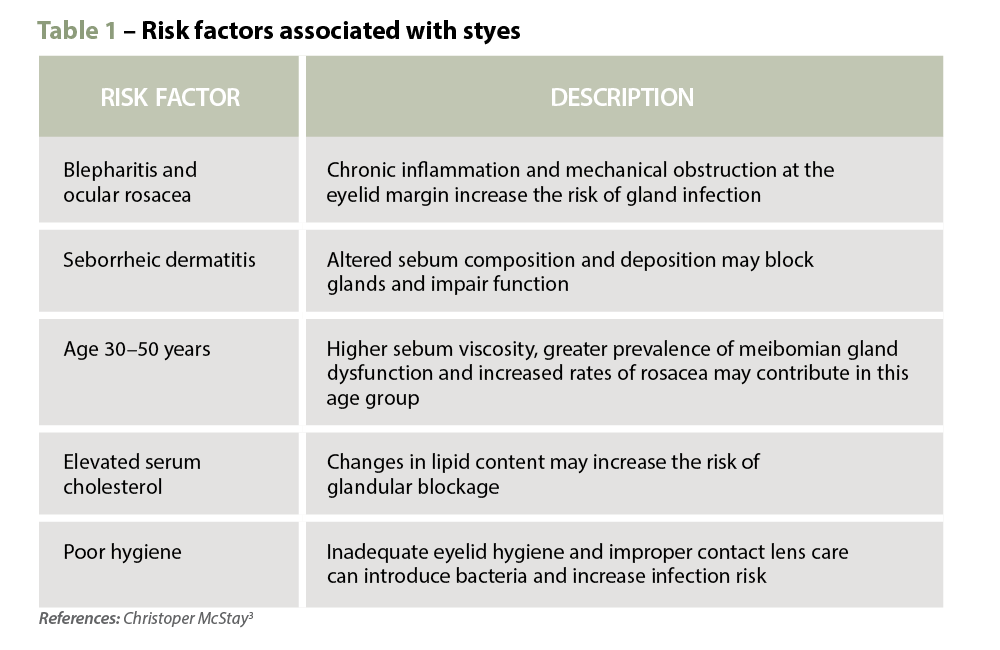 Clinical features
Clinical features 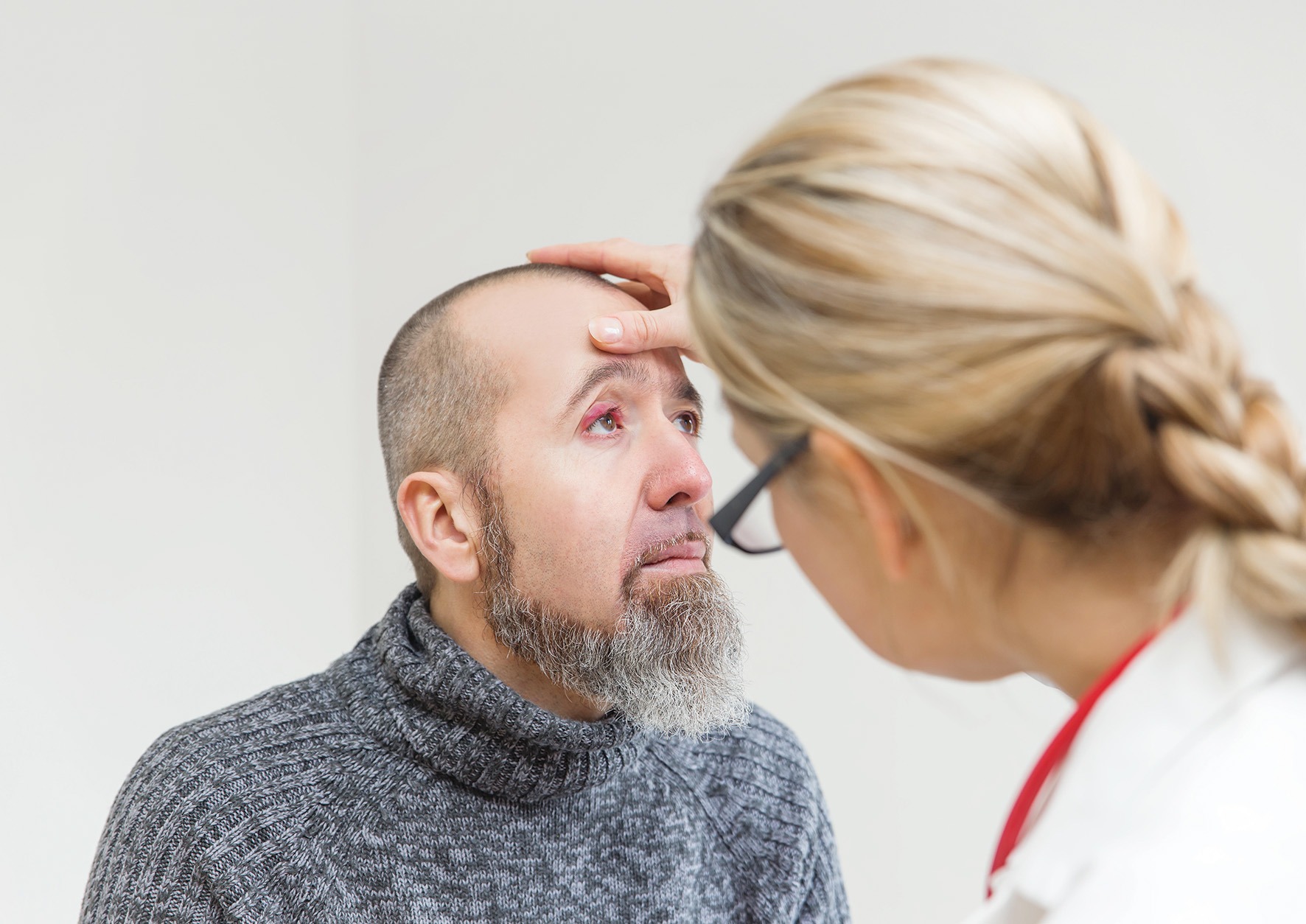 Warm compresses are the cornerstone of treatment, helping to soften the lesion, bring pus to the surface and encourage spontaneous drainage. A clean face cloth soaked in warm (not hot) water should be applied to the closed eyelid for 2–5 minutes, twice daily during the active phase. Once the stye begins to drain, any discharge should be gently wiped away using a clean, warm washcloth. After resolution, continuing warm compresses once daily may help prevent recurrence.2
Warm compresses are the cornerstone of treatment, helping to soften the lesion, bring pus to the surface and encourage spontaneous drainage. A clean face cloth soaked in warm (not hot) water should be applied to the closed eyelid for 2–5 minutes, twice daily during the active phase. Once the stye begins to drain, any discharge should be gently wiped away using a clean, warm washcloth. After resolution, continuing warm compresses once daily may help prevent recurrence.2