Women of reproductive age using glucagon-like peptide-1 receptor agonists (GLP-1 RAs) could be at risk of unintended pregnancy, and may be unaware of associated risks to pregnancy and unborn babies.
A new study by Flinders University, which examined records from more than 1.6 million women aged 18–49 who attended general practice between 2011 and 2022, found that only one in five (21%) of those with first prescribing of GLP-1 RAs had documented contraceptive use.
The study also found that most prescriptions for GLP-1 RAs are now issued to women without diabetes. In 2022 alone, more than 6,000 women began treatment on GLP-1 RAs, and over 90% of those did not have a type 2 diabetes diagnosis.

Participants were tracked at the initial stages of GLP-1 RA therapy, with the research team looking at documented evidence of pregnancies over a 6-month period, said lead author and pharmacist, Associate Professor Luke Grzeskowiak.
‘[While] limited to data from GP records, one in 25 women aged 18 to 34 years had a documented pregnancy at the time of prescribing,’ he said.
‘There will also be pregnancies that the GP might not be aware of, so if anything, what we’re expecting is that this is an underestimate of what’s truly happening.’
Those who were prescribed concurrent contraception were 50% less likely to have a documented pregnancy.
‘So we’ve got clear evidence that contraceptive use at the time of initiating these medicines reduces the risk of pregnancies occurring,’ A/Prof Grzeskowiak said.
Why does GLP-1 RA use increase pregnancy risk?
There are two key reasons: first, it is ‘well established’ that weight loss can improve fertility.
‘Because we know these medicines are very effective at promoting weight loss, it’s highly plausible that they could improve fertility through that mechanism,’ A/Prof Grzeskowiak said.
There have also been concerns that GLP-1 RAs might impact absorption of the oral contraceptive pill.
In June 2025, the UK’s Medicines and Healthcare products Regulatory Agency issued a regulatory warning following case reports of unexpected pregnancies associated with GLP-1 RA use.
‘A detailed review [revealed] that the strongest evidence was around potential interaction between tirzepatide and reduced effectiveness of oral contraception,’ A/Prof Grzeskowiak said.
To date, evidence regarding interactions between GLP-1 RAs and the oral contraceptive pill is limited.
‘So the general recommendations around that regulatory warning were for those relying on oral contraceptive methods to also consider using a barrier method,’ he said.
What are the congenital risk factors?
The research also considered potential harms associated with GLP-1 RAs in pregnancy. Key concerns were taken from a University of Amsterdam review of animal studies, cited in the Flinders University study.
‘In animals, use of GLP-1 RAs [in pregnancy] led to reductions in foetal growth, impairments in bone development and impaired maternal weight gain,’ A/Prof Grzeskowiak said.
At this stage, the human data are more reassuring.
‘The studies that have been done have not shown an increased risk of birth defects,’ he said. ‘But they are still relatively limited in terms of numbers, and we don’t have an examination of the full range of pregnancy outcomes yet,’ he said.
Due to this uncertainty, an abundance of caution is advised.
‘The recommendations are to not use these medicines during pregnancy, and to avoid the potential for them to be used during pregnancy accidentally,’ A/Prof Grzeskowiak said. ‘So it’s important to have a plan around concurrent contraception use, high-quality pre-conception care, and ensure that where pregnancies are planned, everything has been done to optimise pregnancy outcomes.’
What should pharmacists advise patients?
Dispensing GLP-1 RAs provides important opportunities for pharmacists to talk to patients about reproductive health.
For example, when dispensing tirzepatide, access to dispensing data on contraceptive methods enables pharmacists to raise awareness of the potential interaction by initiating an open and unassuming conversation, A/Prof Grzeskowiak said.
‘Having a conversation about how that might be addressed means patients can make an informed decision,’ he said. ‘It might mean changing contraceptive methods or [referring] them back to the GP for a conversation. Or it may be that they’re using contraceptives for non-contraceptive purposes such as acne [management], so there’s a low risk of pregnancy.’
The initiation of therapy is the ideal time to discuss potential risks.
‘That way people know what to expect in terms of the medicines,’ he said.
When commencing GLP-1 RAs, patients may also experience profound gastrointestinal adverse effects, including vomiting or diarrhoea.
‘That in itself can reduce the effectiveness of oral contraception, regardless of any other interactions,’ A/Prof Grzeskowiak said. ‘So people should be aware of the side effects of what to expect when starting this and how it might impact on other treatments that they’re using.’
Pharmacists have an important role in engaging patients in conversations about reproductive health, particularly contraception.
‘Not everyone feels comfortable asking those questions, but there are good training resources, particularly through PSA, around improving pharmacists’ comfort with having those conversations, including around the different types of contraceptive methods,’ he said.
‘It’s one thing to start the conversation, but you also need to be armed with various information to be able to continue it, or at least identify when to refer patients back to their medical practitioner or another [healthcare practitioner] to provide that detailed advice.’



 Team PSA 2026: Caroline Diamantis FPS, Prof Mark Naunton MPS and Bridget Totterman MPS[/caption]
Team PSA 2026: Caroline Diamantis FPS, Prof Mark Naunton MPS and Bridget Totterman MPS[/caption]
 A/Prof Fei Sim and Prof Mark Naunton[/caption]
A/Prof Fei Sim and Prof Mark Naunton[/caption]
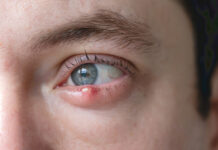
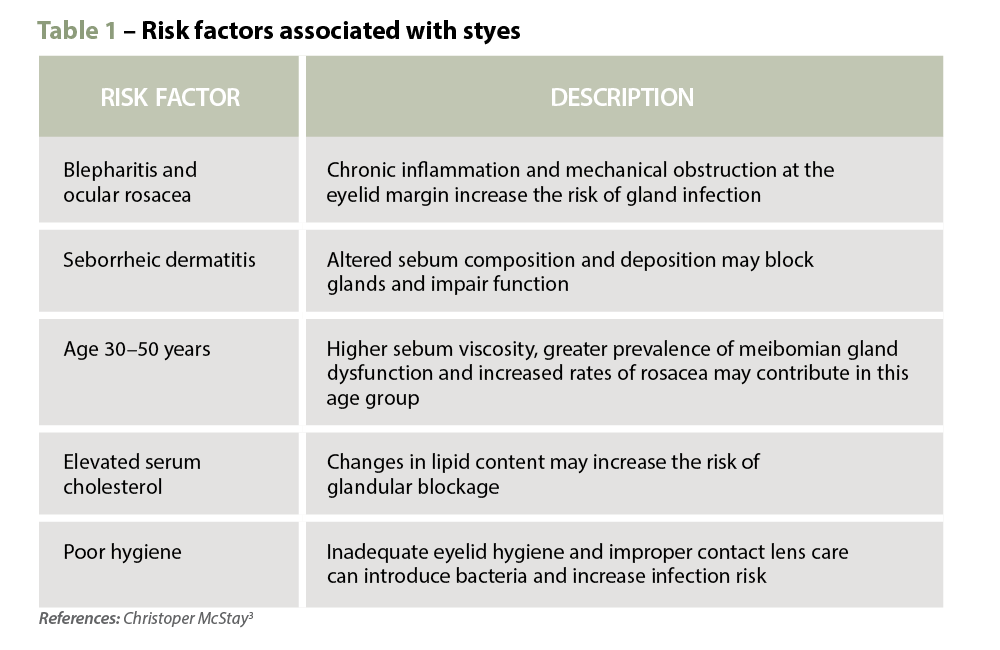 Clinical features
Clinical features 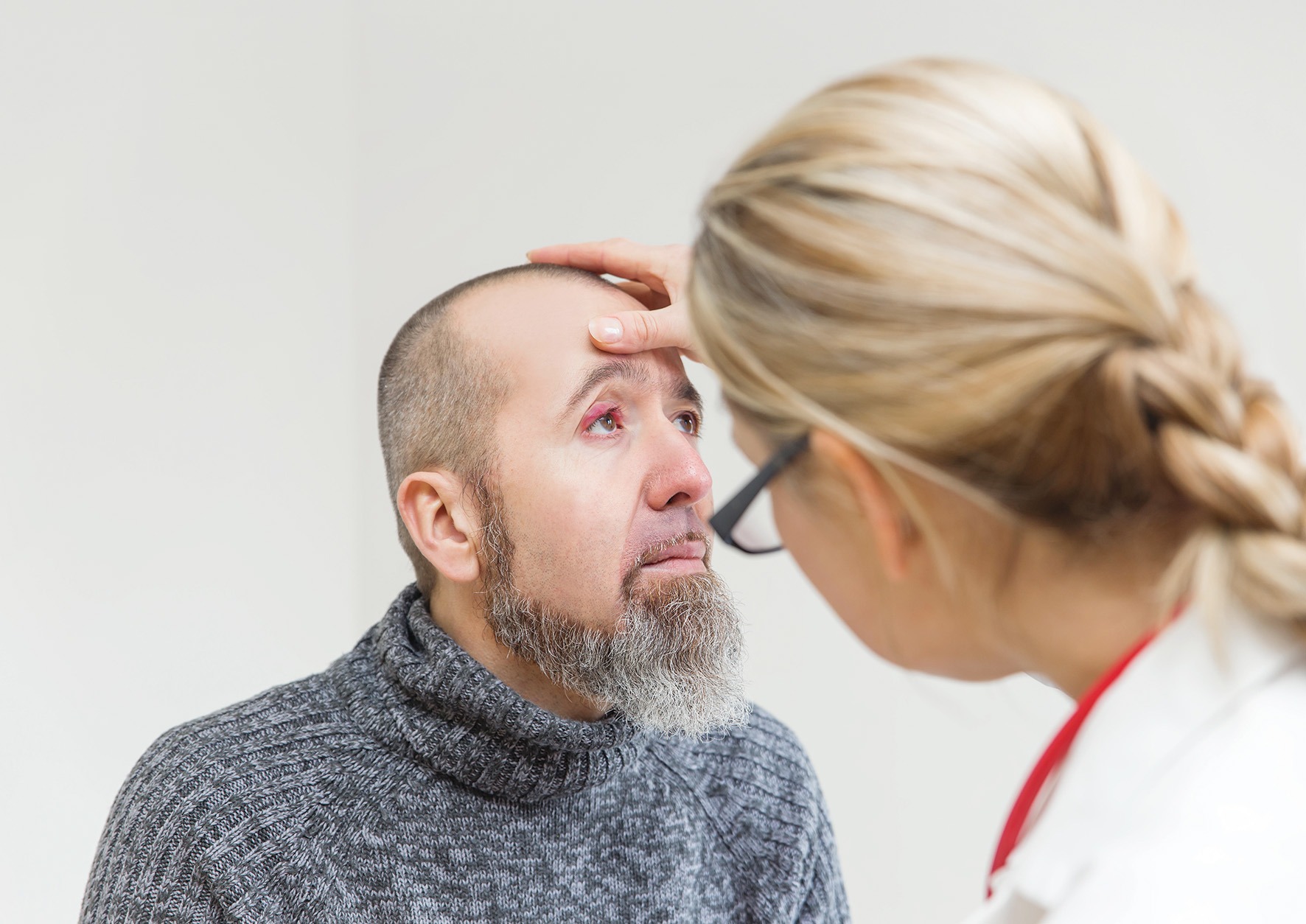 Warm compresses are the cornerstone of treatment, helping to soften the lesion, bring pus to the surface and encourage spontaneous drainage. A clean face cloth soaked in warm (not hot) water should be applied to the closed eyelid for 2–5 minutes, twice daily during the active phase. Once the stye begins to drain, any discharge should be gently wiped away using a clean, warm washcloth. After resolution, continuing warm compresses once daily may help prevent recurrence.2
Warm compresses are the cornerstone of treatment, helping to soften the lesion, bring pus to the surface and encourage spontaneous drainage. A clean face cloth soaked in warm (not hot) water should be applied to the closed eyelid for 2–5 minutes, twice daily during the active phase. Once the stye begins to drain, any discharge should be gently wiped away using a clean, warm washcloth. After resolution, continuing warm compresses once daily may help prevent recurrence.2