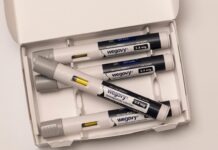
Adrenaline (epinephrine) nasal spray (neffy) was recently approved by the Therapeutic Goods Administration (TGA) and is now available for use in Australia.
The launch of the product marks the first time in over 30 years that a new way of administering adrenaline has been approved in Australia.
So how useful will having a new dose form be? The answer isn’t clear – but the reaction has been positive.
Professor Connie Katelaris AM, a leading NSW allergist welcomes the additional option for care:
‘Anaphylaxis is a difficult condition to manage, with some patients reporting challenges. neffy has been available for some time overseas and now patients in Australia will have access.’
AP explores its place in therapy and whether it’s worth the price.
How does the nasal spray differ from EpiPen?
Similar to EpiPen, neffy delivers adrenaline, the active ingredient used to treat anaphylaxis, a spokesperson for CSL Seqirus, the manufacturer of the medicine, told AP.
‘[However it] doesn’t contain a needle and doesn’t require an injection.’

Using a similar delivery device as opioid reversal medicines, the nasal spray administers adrenaline via the nasal mucosa, enabling rapid absorption into the bloodstream.
Can it be placed in a resuscitation kit?
Yes, and no.
While neffy contains adrenaline and is indicated for the same purpose as injectable adrenaline normally kept as part of a resuscitation kit, there is a key difference.
‘Resuscitation kits generally stock items that are Schedule 3, and neffy is currently a Prescription only (Schedule 4) medication,’ the CSL spokesperson said.
Who is the nasal spray best suited to?
Adrenaline (epinephrine) nasal spray is indicated for emergency treatment of anaphylaxis in patients aged 4 years and older and weighing 15 kg or greater, offering an alternative administration route for those who may be needle-phobic – particularly young children.
The medicine comes in two strengths:
- 1 mg for patients 15–30 kg
- 2 mg for patients ≥ 30kg
What should pharmacists know about its use?
Adrenaline (epinephrine) nasal spray cannot be used in children who are under 4 years of age, and/or are under 15 kg, whereas adrenaline auto‑injectors provide a treatment option in this group, with a 0.15 mg dose typically prescribed for children who weigh between 7.5 kg and 20 kg.
It should be recognised that the presence of any condition that increases the risk of adverse reactions does not contraindicate adrenaline administration in an acute, life-threatening situation. There are absolutely no contraindications to adrenaline in anaphylactic reactions.
Who is most likely to experience adverse effects?
In practice, some patients may be more likely to experience adverse effects with adrenaline (epinephrine) nasal spray, including individuals with raised intraocular pressure, severe renal impairment, prostatic adenoma with residual urine, hypercalcaemia or hypokalaemia. Increased risk may also apply to patients with hyperthyroidism, cardiovascular disease, hypertension or diabetes, as well as older adults and pregnant people. Patients with Parkinson’s disease may experience temporary worsening of symptoms such as rigidity or tremor.
Who might find it hard to use the nasal spray?
The nasal route of administration may present challenges for use in certain patient groups. While clinical studies included people with a history of allergic rhinitis, those with structural or anatomical nasal issues (such as polyps, previous nasal fractures or injuries, or past nasal surgery) were not included. It’s not known how these conditions might affect how well neffy is absorbed.
Could adrenaline nasal spray (neffy) be downscheduled?
Potentially. CSL Seqirus as the product sponsor has submitted an application to the TGA for registration of both the 1 mg and 2 mg as a Schedule 3 medicine, the CSL spokesperson said.
How much does it cost?
The recommended retail price for one box of neffy 1 mg or 2 mg (containing two nasal sprays) is $194.
This is approximately 20% more expensive than EpiPen, excluding doctor consultation fees for a prescription.
Will the nasal spray be PBS listed?
CSL Seqirus has also submitted an application for adrenaline (epinephrine) nasal spray to be subsidised on the Pharmaceutical Benefits Scheme, with the submission being considered in the March 2026 Pharmaceutical Benefits Advisory Committee meeting.
Where can I find more information?
The PSA is in the process of updating the Australian Pharmaceutical Formulary and Handbook adrenaline treatment guideline. Stay tuned for further updates!





 Tahnee Simpson[/caption]
Tahnee Simpson[/caption]
 Nicolette Ellis MPS[/caption]
Nicolette Ellis MPS[/caption]

 Ruth Nona[/caption]
Ruth Nona[/caption]