Why is it that so few blood pressure devices sold online in this country are properly tested for accuracy?
Australian pharmacies are a reliable source of trusted medical products. But less than a quarter of blood pressure (BP) devices sold online by Australian pharmacies have been properly tested for accuracy.1
High BP (hypertension) is the single largest modifiable risk factor contributing to the biggest global burden of disease from cardiovascular events.2 If appropriately identified, hypertension and associated cardiovascular disease risk can be lowered with lifestyle changes or antihypertensive medicines.2 For these reasons accurate BP measurement is a cornerstone medical test.
One of the recommended BP measurement approaches is self-measurement at home.3,4 Australian pharmacies are a major source of supply for consumers seeking to purchase a reliable BP device for this purpose. Home BP measurement is particularly relevant during COVID-19 isolation, with restricted access to health professionals.5
A key factor in measuring BP is ensuring that the BP device has been tested for accuracy (validated) according to internationally approved scientific protocols.6 These tests must be carried out by investigators who are independent from the manufacturer, to avoid potential bias towards positive results.
The recommended protocol for accuracy testing involves multiple comparisons of the test BP device with a reference standard (mercury or non-mercury sphygmomanometry) among 85 people, comprising at least 30% men and women, across different BP levels.6 This protocol is strictly standardised and performed by trained investigators, with results published in the peer-reviewed literature for complete transparency.
BP devices that have not undergone such validation testing are more likely to be inaccurate and lead to inappropriate medical management. This is either by medicine use that is not needed when BP is incorrectly measured as ‘hypertensive’, or failure to provide appropriate therapy to reduce risk for cardiovascular events when BP is incorrectly measured as ‘normotensive.’7
Non-validated BP devices are also more likely to cost less,1 thus potentially creating greater enticement to purchase. So why are these non-validated BP devices allowed to be sold in Australia?
Firstly, this is a global issue not limited to Australia, whereby less than 20% of BP devices worldwide are likely to have been properly validated. In total, there are more than 3,600 unique BP devices sold internationally by more than 450 different companies,8 in a market size projected to be greater than US$2 billion (about A$2.75 billion) by 2025.9
Secondly and critically, there are loopholes that enable the clearance of BP devices for sale by national regulatory authorities (the Therapeutic Goods Administration in Australia) without having undergone the standardised accuracy testing described above. Instead, this testing can be done in-house by the manufacturer using protocols (and results) that are not made publicly available.10 Thus, BP devices can be sold to consumers without having undergone scientifically approved accuracy testing.
The extent to which Australian pharmacists are aware of this problem is not known, but is something the authors are seeking to better understand through a recently completed national survey. We expect pharmacists will be surprised – if not shocked – and will want the problems rectified.
An international project from the Lancet Commission on Hypertension Group in London, United Kingdom, has been initiated to redress these issues, and to increase the global availability of validated BP devices.7 Many schemes towards this goal are underway, including development of a Menzies Institute resource11 to help people find a BP device that has been properly tested for accuracy. This resource provides a step-by-step guide to searching listings of validated BP devices.
Efforts to influence regulatory pathways, as well as national supply chains to ensure favoured access to validated BP devices, are important objectives towards improving the situation. Readers are invited to be part of the solution by ensuring that only validated BP monitors are supplied to the Australian community by pharmacists.
Key points for pharmacists
|
References
- Picone DS, Deshpande R, Schultz MG, et al. Non-validated home blood pressure devices dominate the online marketplace in Australia: major implications for cardiovascular risk management. Hypertension 2020;75:1593–1599. At: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.120.14719
- Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on Hypertension. Lancet 2016;388:2665–2712. At: https://pubmed.ncbi.nlm.nih.gov/27671667/
- Sharman JE, Howes FS, Head GA, et al. Home blood pressure monitoring: Australian expert consensus statement. J Hypertens 2015;33:1721–1728. At: www.ncbi.nlm.nih.gov/pmc/articles/PMC4671913/
- Sharman JE, Howes F, Head G, et al. How to measure home blood pressure: recommendations for healthcare professionals and patients. Aust Fam Physician 2016;45:31–34. At; www.racgp.org.au/afp/2016/januaryfebruary/how-to-measure-home-blood-pressure-recommendations-for-healthcare-professionals-and-patients/
- Sharman JE, Nelson MR, Schlaich M. How to manage your blood pressure in isolation. The Conversation 2020, Apr 23:At: https://theconversation.com/how-to-manage-your-blood-pressure-in-isolation-135958
- Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ ISO) collaboration statement. Hypertension 2018;71:368–374. At: https://pubmed.ncbi.nlm.nih.gov/29384983/
- Sharman JE, O’Brien E, Alpert B, et al. Lancet Commission on Hypertension group position statement on the global improvement of accuracy standards for devices that measure blood pressure. Journal of Hypertension 2020;38:21–29. At: www.ncbi.nlm.nih.gov/pmc/articles/PMC6919228/
- Blood pressure monitors. [Internet] Dublin, Ireland: Medaval Ltd; 2018. At: https://medaval.ie/blood-pressure-monitors/
- Fortune Business Insights. Blood pressure monitoring market size, share and industry analysis by product type (sphygmomanometers, digital blood pressure monitors, ambulatory blood pressure monitors), by end user (hospitals, ambulatory surgery centers & clinics, home healthcare & others), and regional forecast 2018–2025. At: fortunebusinessinsights.com/industry-reports/blood-pressure-monitors-market-100059
- Sharman JE, Padwal R, Campbell NRC. Global marketing and sale of accurate cuff blood pressure measurement devices. Circulation 2020;142:321–323. At: https://pubmed.ncbi.nlm.nih.gov/32718253/
- Menzies Institute. How to check that a blood pressure monitor has been properly tested for accuracy. 2020. At: menzies.utas.edu.au/documents/pdfs/Blood-pressure-devices.pdf
PROFESSOR JAMES SHARMAN is Professor of Medical Research, deputy director of the Menzies Institute for Medical Research and a member of the Lancet Commission on Hypertension Group.
DISTINGUISHED PROFESSOR GREGORY PETERSON BPharm (hons), PhD, MBA, FSHP, FACP, FPS, AACPA is the Professor of Pharmacy at the University of Tasmania.
DR DEAN PICONE is a Postdoctoral Research Fellow in the Blood Pressure Research Group at the Menzies Institute for Medical Research.
PROFESSOR ALETTA SCHUTTE is Principal Theme Lead of Cardiac, Vascular and Metabolic Medicine at the School of Population Health, University of New South Wales, a Professorial Fellow at the George Institute for Global Health, Sydney, and a member of the Lancet Commission on Hypertension Group.
DR SHANE JACKSON BPharm, PhD, FPS, AACPA, AdvPracPharm is a Clinical Senior Lecturer in Pharmacy at the University of Tasmania.




 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.

 Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4
Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4  C – Comorbidity and risk factor management
C – Comorbidity and risk factor management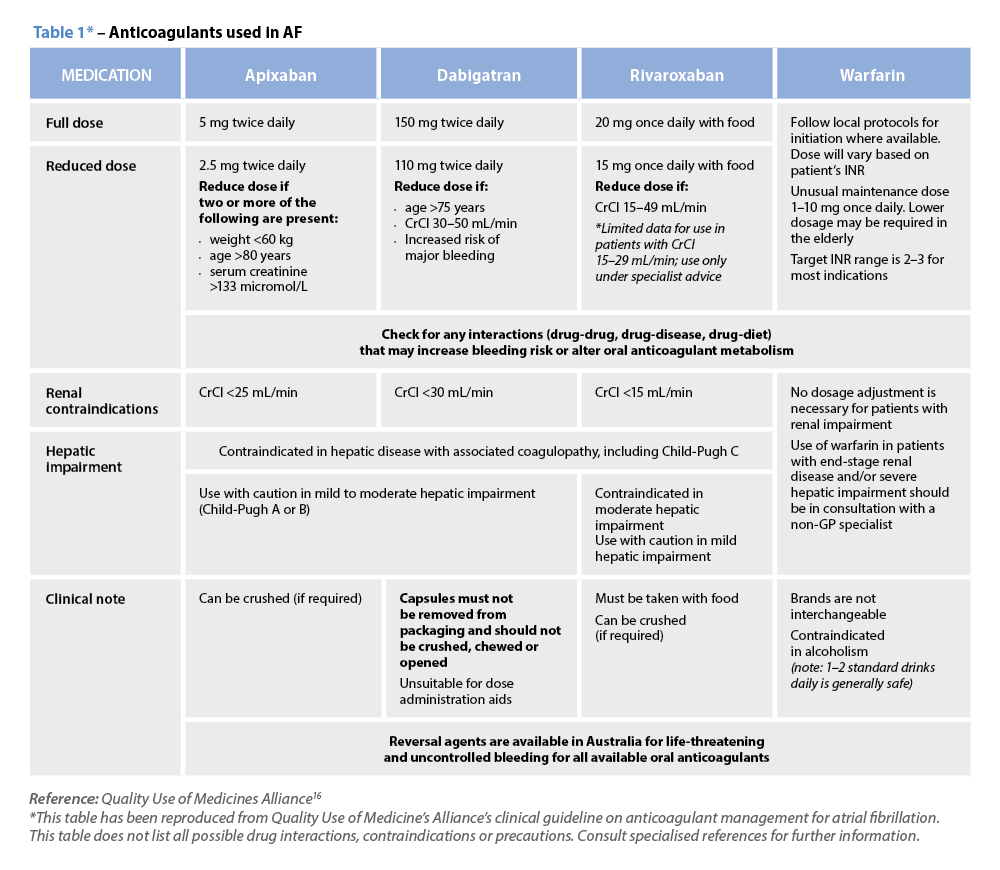 Warfarin
Warfarin







