Can oxybutynin be useful for some patients who are unsuited for hormonal pharmacotherapy?
Some people might describe hot flashes as an unannounced and brief feeling of intense heat, with the sensation that their body is on fire and they are about to combust. Hot flashes can last from 30 seconds to a few minutes, and can occur once a day, to much more frequently e.g. every hour. They tend to start as a sudden feeling of heat on the upper chest and face that quickly spreads, and is associated with profuse sweating, flushing and palpitations. This is then sometimes followed by chills and shivering as the body tries to regulate and restore the core temperature.
Hot flashes are most commonly experienced by women in menopause (up to 80%), but other medical conditions can be responsible for causing hot flashes e.g. in breast cancer survivors after treatment. Hot flashes are thought to be related to oestrogen withdrawal, leading to a thermoregulatory dysregulation at the hypothalamus – but this only occurs after the person has been exposed to adult levels of oestrogen. Interestingly, low oestrogen alone does not appear sufficient for causing hot _ ashes as these are not experienced by prepubertal girls with low oestrogen levels. As such, hot _ ashes are often managed by administration of oestrogen. However, not everyone can use hormone replacement therapy e.g. women with, or a history of breast cancer. Unfortunately, these women also tend to be more affected by hot _ ashes because they become oestrogen deficient quite abruptly due to chemotherapy causing ovarian failure, or bilateral oophorectomies. Additionally, antioestrogen therapies (e.g. tamoxifen, when used either as treatment, adjuvant or for chemoprophylaxis) can cause hot flashes in many women.
Options for non-hormonal pharmacotherapy include low-dose antidepressants (venlafaxine or paroxetine), gabapentin or clonidine. It is not clear why venlafaxine (a serotonin and noradrenaline reuptake inhibitor) and paroxetine (a selective serotonin reuptake inhibitor) help with hot _ ashes. This is also the case with gabapentin, which works by reducing calcium influx and neurotransmitter release by binding to the alpha-2 delta protein subunit of high threshold voltage-dependent calcium channels.
There is only limited evidence from short-term studies (<12 weeks) supporting the use of these medicines for reducing the frequency and severity of hot flashes, and more work is needed to better understand their clinical utility. Of the non-hormonal pharmacotherapies, clonidine (a centrally acting agonist at alpha2 adrenoreceptors, and imidazoline receptors) is the only one with a marketed indication for menopausal flushing. However, side effects, limited evidence for efficacy, and the emergence of other non-hormonal pharmacotherapies also limits the usefulness of clonidine.
Therefore, the search is still on for non-hormonal pharmacotherapies to help with hot flashes. In trials with oxybutynin for controlling muscle spasms, decreased sweating was noted as a side effect – presumably due to its anticholinergic effects. As such, more work was conducted to explore the usefulness of oxybutynin for hot flashes. Initial studies looked at oxybutynin at doses similar to that used for urinary incontinence, but found many patients discontinued use due to side effects.
TABLE 1. Oxybutynin (oral) dosing information (AMH)
| CONDITION | DOSING (ADULT) |
| Urinary urge incontinence | Oral, usual range 2.5–5 mg 2 or 3 times daily; maximum 20 mg daily. For elderly patients, start with 2.5 mg at night; if necessary, increase dose slowly. |
| Hot flashes* | 2.5 mg twice daily, with the option of a subsequent increase to 5 mg twice daily. |
*Oxybutynin does not have an indication for this condition – dose provided is indicative only
More recently, a randomised placebo-controlled clinical trial looked at using lower doses of oxybutynin i.e. 2.5 mg twice daily for 6 weeks, or 2.5 mg twice daily for 1 week before increasing to 5 mg twice daily. Interim results were reported for 104 participants (out of the 146 women who were recruited into the study). These women experienced at least 28 episodes of hot flashes per week, for more than 30 days, with the symptoms being severe enough to seek treatment. The primary endpoint measured was the change in weekly hot flashes score, as measured using the Hot Flash Related Daily Interference Scale (HFRDIS), and frequency of hot flashes compared to baseline. Both doses of oxybutynin significantly reduced the hot flash score and frequency of episodes (<0.001). The lower oxybutynin dose, compared to the placebo, saw a mean change in hot flash score of -10 vs -5.1 (p=0.003), and a mean change in frequency of episodes each week -4.6 vs -2.3 (p=0.002), respectively. More significant changes were noted at the higher oxybutynin dose compared to the placebo, with the mean change in hot flash score of -16.2 vs -5.1 (p<0.001), and a mean change in frequency of episodes each week -7 vs -2.3 (p<0.001), respectively.
Unsurprisingly, more side effects were reported for the oxybutynin arms compared to placebo, and side effects were those expected of anticholinergic medicines. Nevertheless, there were no differences in patients withdrawing from the study due to adverse effects when compared to the placebo (p=0.653 and p=0.483) for both the lower and higher oxybutynin doses respectively.
Conclusion
While these are only interim results, oxybutynin may be useful for some patients who are unsuited for hormonal pharmacotherapy. Certainly, much more work is required e.g. more participants, with longer follow-up periods, to help determine the clinical utility of oxybutynin for hot flashes. This is particularly important given the well-known issues and side effects associated with the use of anticholinergic medicines.
References
- Leon-Ferre RA, Novotny PJ, Faubion SS, Ruddy KJ, Flora D, Dakhil C, Rowland KM, GrahamML, Le-Lindqwister N, Loprinzi CL. A randomized, double-blind, placebo-controlled trial of oxybutynin (Oxy) for hot flashes (HF): ACCRU study SC-1603. In: Proceedings of the San Antonio Breast Cancer Symposium; 2018 Dec 7. Texas (US). Available from: https://www.abstracts2view.com/sabcs18/view.php?nu=SABCS18L_1448
- Leon-Ferre RA, Majithia N, Loprinzi CL. Management of hot flashes in women with breast cancer receiving ovarian function suppression. Cancer Treat Rev. 2017;52:82–90. At: https://www.ncbi.nlm.nih.gov/pubmed/27960127
- Faubion SS, Loprinzi CL, Ruddy KJ. Management of Hormone Deprivation Symptoms After Cancer. Mayo Clin Proc. 2016 Aug;91(8):1133–46. At: https://www.mayoclinicproceedings.org/article/S0025-6196(16)30117-3/pdf
- Simon JA, Gaines T, LaGuardia KD. Extended-release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial. Menopause. 2016 Nov;23(11): 1214–21. At: https://www.ncbi.nlm.nih.gov/pubmed/27760081
- Sexton T, Younus J, Perera F, Kligman L, Lock M. Oxybutynin for refractory hot flashes in cancer patients. Menopause. 2007;14(3 Pt 1):505–9. At: https://www.ncbi.nlm.nih.gov/pubmed/17204995
- Smith TJ, Loprinzi CL, Deville C. Oxybutynin for Hot Flashes Due to Androgen Deprivation in Men. N Engl J Med. 2018;378(18):1745–6. At: https://www.ncbi.nlm.nih.gov/pubmed/29719180






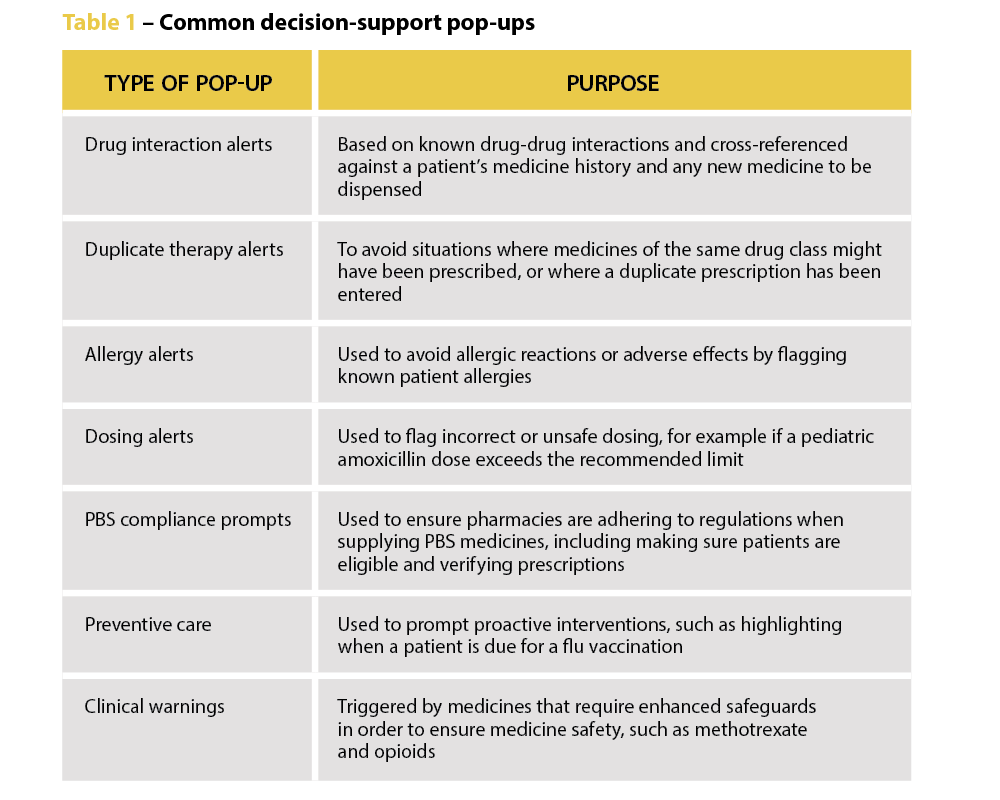 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.