The social highlight of the annual PSA conference is the Gala Dinner, and this year certainly did not disappoint.


td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31334
[post_author] => 3410
[post_date] => 2026-02-11 14:25:30
[post_date_gmt] => 2026-02-11 03:25:30
[post_content] => Victoria recently announced ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
But last week, the Allan Government also unveiled a nation-first initiative. From September, an online emergency prescribing pathway will allow people with an existing ADHD diagnosis to obtain urgent repeat medicine through the Victorian Virtual Emergency Department (VVED).
AP explores what the new model involves, the safeguards that will be in place and what’s happening in other states and territories.
What’s the purpose of the service?
The telehealth service is designed to address growing concern about long specialist waitlists, escalating costs and the clinical risks associated with abrupt cessation of Schedule 8 ADHD medicines – which can lead to symptom rebound, functional impairment and significant distress.
A Department of Health spokesperson told AP that the service would provide a targeted safety net, rather than a substitute for established care arrangements.
‘The Victorian Virtual Emergency Department will offer a safe way for Victorians with an existing ADHD diagnosis to refill an urgent prescription for ADHD medication,’ the spokesperson said.
How will urgent ADHD ‘repeats’ be issued?
Clinicians working within the VVED will verify the patient’s current medicine and dosage before issuing a prescription, the spokesperson said.
Scripts will be sent directly to the patient’s local pharmacy, with patients advised of the closest pharmacy in operation at the time of prescribing.
The Department of Health emphasised that the pathway will not replace routine ADHD management.
‘This is for emergency situations only and will not replace the important ongoing treatment and relationship between a patient and their clinician,’ the spokesperson said.
Who will be eligible?
The Victorian model allows adults and children aged 6 and over with an existing ADHD diagnosis to access the service who cannot secure a timely appointment with their usual clinician.
The service will be limited to people who are already prescribed ADHD medicines.
The VVED will not initiate ADHD treatment, alter dosages or provide ongoing prescribing.
What are the safeguards?
Existing regulatory requirements and clinical guidelines for ADHD medicines will remain fully in place under the VVED pathway.
‘The clinicians at the VVED, including paediatricians and psychiatrists, are highly experienced and highly skilled,’ the spokesperson said. ‘They will prescribe the medication within their existing scope of practice and clinical operations.’
Mandatory use of SafeScript for Schedule 8 medicines will continue to operate as a core safeguard. This ensures prescribers and pharmacists can monitor dispensing histories and reduces the risk of patients obtaining excessive prescriptions from multiple clinicians.
The Department has stressed that responsibility for ongoing ADHD management remains with the patient’s regular clinician, with the VVED acting solely as a one-off support mechanism that complements broader reforms aimed at expanding GP involvement in ADHD care.
And rather than providing a script to the patient, the script will be sent directly to their local pharmacy. When issuing a script, the VVED advises the patient of the closest pharmacy in operation.
Part of a broader national shift
Victoria’s online emergency model sits within a wider national trend to rebalance ADHD care away from exclusive reliance on specialist services.
Since 1 December 2025, ‘specialist GPs' in Queensland have been able to initiate, modify and continue stimulant treatment for adults with ADHD under updated Queensland Health guidance.
Today (11 February), ACT Health issued an announcement on ADHD prescribing, with GPs who have completed approved training now able to continue prescribing ADHD medicines for eligible patients without requiring repeated reviews from a psychiatrist, paediatrician or neurologist.
And other jurisdictions have since followed suit, including New South Wales, Western Australia and South Australia – which are set to roll out similar reforms this year.
Across Australia, governments are seeking to reduce wait times, lower out-of-pocket costs and embed ADHD care more firmly within primary care, while maintaining strong oversight of Schedule 8 stimulants such as methylphenidate, dexamfetamine and lisdexamfetamine via authorised prescribing schemes and real-time prescription monitoring checks. Non-stimulant ADHD medicines remain Schedule 4 and continue to be prescribed under existing arrangements.
For more information, complete the PSA online module: ADHD explained.
[post_title] => What pharmacists need to know about emergency prescribing for ADHD
[post_excerpt] => Victoria is the latest state to announce ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => what-pharmacists-need-to-know-about-emergency-adhd-prescribing
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-11 15:45:52
[post_modified_gmt] => 2026-02-11 04:45:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31334
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => What pharmacists need to know about emergency prescribing for ADHD
[title] => What pharmacists need to know about emergency prescribing for ADHD
[href] => https://www.australianpharmacist.com.au/what-pharmacists-need-to-know-about-emergency-adhd-prescribing/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31336
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31316
[post_author] => 3410
[post_date] => 2026-02-09 12:57:25
[post_date_gmt] => 2026-02-09 01:57:25
[post_content] => The gap between evidence and everyday practice is impacting patient access to emergency contraception.
Community pharmacies are often the first – and sometimes only – point of access for emergency contraception in Australia.
But a new qualitative study found there are still persistent gaps between what the guidelines say and what happens in practice.
Ruth Nona, pharmacist and researcher at James Cook University, who interviewed community pharmacists about providing emergency contraception services, describes a workforce that is broadly willing to help, but not always set up to deliver consistently equitable care.
1. Not recommending ulipristal as first-line
One of the most significant gaps identified in the study was the tendency for pharmacists to default to levonorgestrel, despite guidance in the Australian Pharmaceutical Formulary and Handbook (APF) that ulipristal acetate is generally considered more effective than levonorgestrel and can be used up to 120 hours after unprotected intercourse.
‘Habit definitely plays a role,’ Ms Nona said. ‘Some pharmacists felt more comfortable and confident supplying levonorgestrel. For example, if someone requested emergency contraception within 24 hours, pharmacists felt levonorgestrel was acceptable within that timeframe, without fully considering efficacy.’
[caption id="attachment_31329" align="alignright" width="250"] Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
‘If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information.'
Ruth nona
2. Uncertainty when responding to third-party requests
Pharmacists reported particular uncertainty when providing emergency contraception to third parties, with many wary about consent and unsure whether they could provide it to someone who wasn’t the patient.
‘In most cases, they would follow up and ask to contact the patient directly,’ Ms Nona said. ‘It wasn’t that they didn’t want to provide it – they just felt unsure and they wanted to make sure that the patient had consented.’
While in many cases, speaking to the intended person directly can help provide key information and counselling, in circumstances where this is not possible, it is usually possible to determine the medicine is safe and therapeutically appropriate, and supply in a manner consistent with APF guidance and legal obligations.
3. Uncertainty providing emergency contraception to adolescents
Similarly, pharmacists were ‘uncomfortable’ providing the service to adolescents.
This uncertainty often centred on fear of making the wrong decision or facing professional consequences.
‘They still wanted to provide the service, but it was more that internal question of, “Should I do this? Am I going to get in trouble?”’
There are no jurisdictions where there’s a legal restriction from supplying emergency contraception to minors. The APF guidance helps pharmacists navigate Gillick competency and consent in adolescents, ensuring they demonstrate sufficient maturity and understanding to provide informed consent.
Ms Nona emphasised that the issue was confidence, not capability.
‘It’s about being familiar with the guidelines, making sure we’re as up to date as possible and realising that it’s okay for us to provide these services, provided the adolescent is [assessed as Gillick competent] mentally mature and safe,’ she said.
The APF references Gillick competency provided the adolescent is [assessed as Gillick competent (demonstrating sufficient maturity and understanding to provide informed consent)]
4. Uncertainty for transgender people on gender affirming hormone therapy
While pharmacists were generally supportive towards transgender and gender-diverse patients, lack of familiarity with hormone therapy raised hesitation.
‘There are absolutely no interactions between emergency contraception and gender-affirming hormones’ Ms Nona said. ‘It really comes down to knowledge, which builds confidence, and being up to date to make sure the service we provide is timely and equitable.’
In some areas, pharmacists may frequently encounter transgender and gender- diverse patients requesting emergency contraception, while pharmacists in other areas do not.
‘That’s why it’s also about being prepared. You never know when that situation might arise,’ she added.
Should pharmacists feel unsure during these consultations, pharmacists can and should engage with the APF.
‘Pharmacists did say that if that situation did occur with a transgender or gender- diverse person that they would be honest and say to them, “Do you mind if I consult my resources?”’
Another reason the APF is a mandatory text for all community pharmacists
Despite lack of guideline use, pharmacists acknowledged how essential guidelines
such as the APF are, Ms Nona said.
‘And when pharmacists did use them, they found the information provided was invaluable.’
‘[But] a lot of the challenges stemmed from lack of time and, in some cases, a lack of up-to-date knowledge. We have so many things to do, and we need more time to do everything and to keep ourselves up to date.’
For Ms Nona, the solution lies in supporting pharmacists to use guidelines confidently and consistently in real-world conditions.
Some pharmacists report to PSA that they will often bring up the APF digital on the screen in the consultation room in emergency contraception discussions, particularly in situations which are new or unfamiliar.
Delivering a critical intervention
The key to emergency contraception provision is recognising the stakes.
‘The whole picture of providing emergency contraception is to make sure we are preventing pregnancies when people don't want to get pregnant – whatever the reason may be,’ Ms Nona said. ‘That’s why they come to see a pharmacist – to ensure the person has the best possible chance of preventing an unintended pregnancy.’
When pharmacists are supported to provide full information and informed choice, patients respond accordingly.
The Australian Pharmaceutical Formulary and Handbook (APF) chapter on ‘Emergency Contraception’, provides essential guidance on:
|
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31305
[post_author] => 1925
[post_date] => 2026-02-08 10:38:16
[post_date_gmt] => 2026-02-07 23:38:16
[post_content] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
The range of professional services delivered by community pharmacists has expanded rapidly in recent years, from vaccination to UTI prescribing and beyond. As
these services increase in popularity, they are shifting from ancillary service to core business.
This widening scope is forcing community pharmacies to review how they conduct their business and the way front-of-house staff interact with patients.
No longer is dispensing prescriptions on a first come, first served basis sustainable. With adjustments to workflow, vaccinations and other booked services have been prioritised and run simultaneously, says Queensland-based prescribing pharmacist Kate Gunthorpe MPS.
‘We are moving away from the mindset that dispensing always comes first. We need to triage effectively and manage expectations, so every patient feels seen and cared for,’ she says.
And it isn’t just about sequential processes. Workflow changes also require a shift of communication approaches and pre-existing mindsets around professional service provision.
‘The biggest pitfall I’ve discovered is apologising for charging or determining that the consultation wasn’t worth charging for,’ Ms Gunthorpe says. ‘That instantly undermines the service’s value. Every consultation, whether the outcome is a prescription, advice or reassurance, involves clinical reasoning, professional judgement and patient care.’
So, how should the profession move forward? The PSA’s foundation documents are clear that all services must remain patient-centric.
That means redesigning workflows on the floor, developing new communication strategies for staff and providing additional training for pharmacy assistants to ensure a consistent, professional patient experience.
AP spoke with Ms Gunthorpe and pharmacy assistant Madison Low about adapting workflow to integrate services without disrupting dispensing. product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
Case 1 Kate Gunthorpe MPS
Pharmacist prescriber, Implementation and Change Specialist, TerryWhite Chemmart, Samford, Queensland
[caption id="attachment_31312" align="alignright" width="185"] Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Case 2 Madison Low
Retail manager, TerryWhite Chemmart, Arana Hills, Queensland
[caption id="attachment_31313" align="alignright" width="277"] Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31266
[post_author] => 11883
[post_date] => 2026-02-04 13:01:11
[post_date_gmt] => 2026-02-04 02:01:11
[post_content] => Case scenario
Greg, a 28-year-old man, comes into your pharmacy asking for a ‘strong minoxidil hair product’. He explains that his doctor recently diagnosed him with male pattern hair loss and suggested he try an over-the-counter treatment, with a follow-up review in 6 months. Greg has noticed gradual thinning at the temples over the past year but reports no sudden hair loss, scalp irritation or other medical issues. He has no known allergies, takes no medicines and has no chronic conditions.
Learning objectivesAfter reading this article, pharmacists should be able to:
|
Case scenario continuedAfter reviewing Greg’s history, you confirm there are no contraindications to minoxidil therapy. You explain the correct use of an over-the-counter foam formulation: applying to a dry scalp, taking care around the forehead and temples, waiting at least 1 hour before using other products and avoiding washing for 4 hours after application. You discuss the treatment timeline, reassuring Greg that initial shedding may increase but usually settles, and that it can take 3–4 months of consistent use before improvement is noticeable. You also emphasise the importance of follow-up with his doctor in 6 months and suggest simple supportive measures, such as protecting the scalp from sun exposure. Throughout the conversation, you address Greg’s concerns, reinforce realistic expectations, and encourage adherence to achieve the best outcome.1,2,8 |
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31273
[post_author] => 11884
[post_date] => 2026-02-04 12:59:14
[post_date_gmt] => 2026-02-04 01:59:14
[post_content] => Case scenario
Alicia, 27, visits your pharmacy regularly for naproxen and heat patches to manage period pain. She confides that her pain has worsened over the past 2 years, radiates down her legs, interferes with work and affects intimacy.
Her periods are heavy, lasting around 9 days, and leave her feeling exhausted and sometimes even bedridden. Alicia has seen several GPs, who told her it was ‘normal for your age’. She says, ‘It feels like someone’s wringing out my insides – nothing helps much. Is this really normal?’
Learning objectivesAfter reading this article, pharmacists should be able to:
|
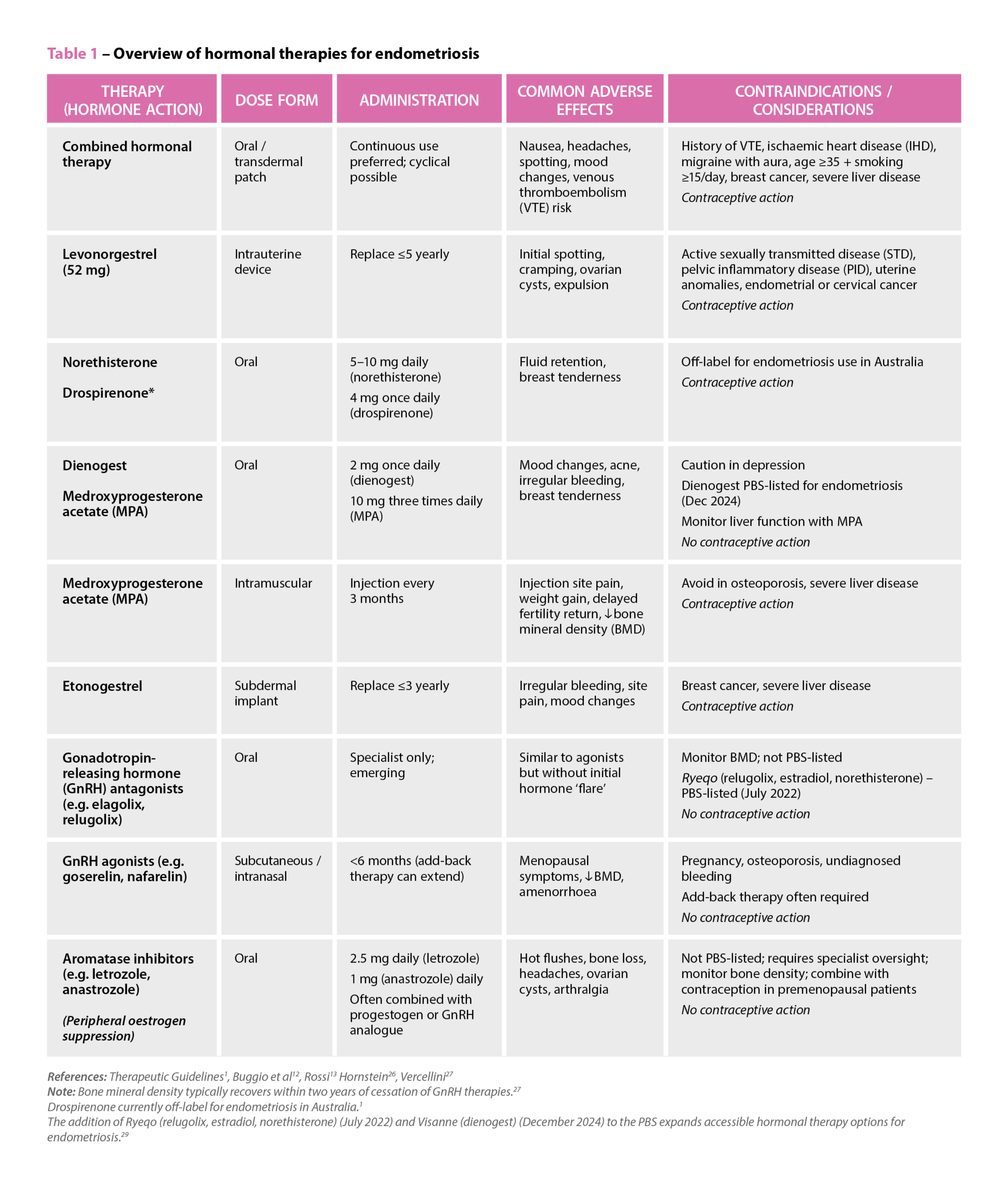 References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
Case scenario continuedYou reassure Alicia that severe period pain is not something she has to accept and suggest tracking her symptoms with a menstrual diary and consulting a women’s health GP. You also provide advice on safe NSAID use and non-pharmacological strategies. Alicia returns 2 months later, now diagnosed with endometriosis and receiving hormonal therapy and pelvic physiotherapy. She continues to experience chronic pelvic pain and questions her medicines, so you organise a Home Medicines Review, identifying potential naproxen overuse and interactions with her sertraline, prompting treatment adjustments. You also recommend a local endometriosis support group, which Alicia joins, and she has since referred two friends with similar symptoms. Through ongoing support, she feels more empowered to manage her condition. |
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31334
[post_author] => 3410
[post_date] => 2026-02-11 14:25:30
[post_date_gmt] => 2026-02-11 03:25:30
[post_content] => Victoria recently announced ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
But last week, the Allan Government also unveiled a nation-first initiative. From September, an online emergency prescribing pathway will allow people with an existing ADHD diagnosis to obtain urgent repeat medicine through the Victorian Virtual Emergency Department (VVED).
AP explores what the new model involves, the safeguards that will be in place and what’s happening in other states and territories.
What’s the purpose of the service?
The telehealth service is designed to address growing concern about long specialist waitlists, escalating costs and the clinical risks associated with abrupt cessation of Schedule 8 ADHD medicines – which can lead to symptom rebound, functional impairment and significant distress.
A Department of Health spokesperson told AP that the service would provide a targeted safety net, rather than a substitute for established care arrangements.
‘The Victorian Virtual Emergency Department will offer a safe way for Victorians with an existing ADHD diagnosis to refill an urgent prescription for ADHD medication,’ the spokesperson said.
How will urgent ADHD ‘repeats’ be issued?
Clinicians working within the VVED will verify the patient’s current medicine and dosage before issuing a prescription, the spokesperson said.
Scripts will be sent directly to the patient’s local pharmacy, with patients advised of the closest pharmacy in operation at the time of prescribing.
The Department of Health emphasised that the pathway will not replace routine ADHD management.
‘This is for emergency situations only and will not replace the important ongoing treatment and relationship between a patient and their clinician,’ the spokesperson said.
Who will be eligible?
The Victorian model allows adults and children aged 6 and over with an existing ADHD diagnosis to access the service who cannot secure a timely appointment with their usual clinician.
The service will be limited to people who are already prescribed ADHD medicines.
The VVED will not initiate ADHD treatment, alter dosages or provide ongoing prescribing.
What are the safeguards?
Existing regulatory requirements and clinical guidelines for ADHD medicines will remain fully in place under the VVED pathway.
‘The clinicians at the VVED, including paediatricians and psychiatrists, are highly experienced and highly skilled,’ the spokesperson said. ‘They will prescribe the medication within their existing scope of practice and clinical operations.’
Mandatory use of SafeScript for Schedule 8 medicines will continue to operate as a core safeguard. This ensures prescribers and pharmacists can monitor dispensing histories and reduces the risk of patients obtaining excessive prescriptions from multiple clinicians.
The Department has stressed that responsibility for ongoing ADHD management remains with the patient’s regular clinician, with the VVED acting solely as a one-off support mechanism that complements broader reforms aimed at expanding GP involvement in ADHD care.
And rather than providing a script to the patient, the script will be sent directly to their local pharmacy. When issuing a script, the VVED advises the patient of the closest pharmacy in operation.
Part of a broader national shift
Victoria’s online emergency model sits within a wider national trend to rebalance ADHD care away from exclusive reliance on specialist services.
Since 1 December 2025, ‘specialist GPs' in Queensland have been able to initiate, modify and continue stimulant treatment for adults with ADHD under updated Queensland Health guidance.
Today (11 February), ACT Health issued an announcement on ADHD prescribing, with GPs who have completed approved training now able to continue prescribing ADHD medicines for eligible patients without requiring repeated reviews from a psychiatrist, paediatrician or neurologist.
And other jurisdictions have since followed suit, including New South Wales, Western Australia and South Australia – which are set to roll out similar reforms this year.
Across Australia, governments are seeking to reduce wait times, lower out-of-pocket costs and embed ADHD care more firmly within primary care, while maintaining strong oversight of Schedule 8 stimulants such as methylphenidate, dexamfetamine and lisdexamfetamine via authorised prescribing schemes and real-time prescription monitoring checks. Non-stimulant ADHD medicines remain Schedule 4 and continue to be prescribed under existing arrangements.
For more information, complete the PSA online module: ADHD explained.
[post_title] => What pharmacists need to know about emergency prescribing for ADHD
[post_excerpt] => Victoria is the latest state to announce ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => what-pharmacists-need-to-know-about-emergency-adhd-prescribing
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-11 15:45:52
[post_modified_gmt] => 2026-02-11 04:45:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31334
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => What pharmacists need to know about emergency prescribing for ADHD
[title] => What pharmacists need to know about emergency prescribing for ADHD
[href] => https://www.australianpharmacist.com.au/what-pharmacists-need-to-know-about-emergency-adhd-prescribing/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31336
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31316
[post_author] => 3410
[post_date] => 2026-02-09 12:57:25
[post_date_gmt] => 2026-02-09 01:57:25
[post_content] => The gap between evidence and everyday practice is impacting patient access to emergency contraception.
Community pharmacies are often the first – and sometimes only – point of access for emergency contraception in Australia.
But a new qualitative study found there are still persistent gaps between what the guidelines say and what happens in practice.
Ruth Nona, pharmacist and researcher at James Cook University, who interviewed community pharmacists about providing emergency contraception services, describes a workforce that is broadly willing to help, but not always set up to deliver consistently equitable care.
1. Not recommending ulipristal as first-line
One of the most significant gaps identified in the study was the tendency for pharmacists to default to levonorgestrel, despite guidance in the Australian Pharmaceutical Formulary and Handbook (APF) that ulipristal acetate is generally considered more effective than levonorgestrel and can be used up to 120 hours after unprotected intercourse.
‘Habit definitely plays a role,’ Ms Nona said. ‘Some pharmacists felt more comfortable and confident supplying levonorgestrel. For example, if someone requested emergency contraception within 24 hours, pharmacists felt levonorgestrel was acceptable within that timeframe, without fully considering efficacy.’
[caption id="attachment_31329" align="alignright" width="250"] Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
‘If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information.'
Ruth nona
2. Uncertainty when responding to third-party requests
Pharmacists reported particular uncertainty when providing emergency contraception to third parties, with many wary about consent and unsure whether they could provide it to someone who wasn’t the patient.
‘In most cases, they would follow up and ask to contact the patient directly,’ Ms Nona said. ‘It wasn’t that they didn’t want to provide it – they just felt unsure and they wanted to make sure that the patient had consented.’
While in many cases, speaking to the intended person directly can help provide key information and counselling, in circumstances where this is not possible, it is usually possible to determine the medicine is safe and therapeutically appropriate, and supply in a manner consistent with APF guidance and legal obligations.
3. Uncertainty providing emergency contraception to adolescents
Similarly, pharmacists were ‘uncomfortable’ providing the service to adolescents.
This uncertainty often centred on fear of making the wrong decision or facing professional consequences.
‘They still wanted to provide the service, but it was more that internal question of, “Should I do this? Am I going to get in trouble?”’
There are no jurisdictions where there’s a legal restriction from supplying emergency contraception to minors. The APF guidance helps pharmacists navigate Gillick competency and consent in adolescents, ensuring they demonstrate sufficient maturity and understanding to provide informed consent.
Ms Nona emphasised that the issue was confidence, not capability.
‘It’s about being familiar with the guidelines, making sure we’re as up to date as possible and realising that it’s okay for us to provide these services, provided the adolescent is [assessed as Gillick competent] mentally mature and safe,’ she said.
The APF references Gillick competency provided the adolescent is [assessed as Gillick competent (demonstrating sufficient maturity and understanding to provide informed consent)]
4. Uncertainty for transgender people on gender affirming hormone therapy
While pharmacists were generally supportive towards transgender and gender-diverse patients, lack of familiarity with hormone therapy raised hesitation.
‘There are absolutely no interactions between emergency contraception and gender-affirming hormones’ Ms Nona said. ‘It really comes down to knowledge, which builds confidence, and being up to date to make sure the service we provide is timely and equitable.’
In some areas, pharmacists may frequently encounter transgender and gender- diverse patients requesting emergency contraception, while pharmacists in other areas do not.
‘That’s why it’s also about being prepared. You never know when that situation might arise,’ she added.
Should pharmacists feel unsure during these consultations, pharmacists can and should engage with the APF.
‘Pharmacists did say that if that situation did occur with a transgender or gender- diverse person that they would be honest and say to them, “Do you mind if I consult my resources?”’
Another reason the APF is a mandatory text for all community pharmacists
Despite lack of guideline use, pharmacists acknowledged how essential guidelines
such as the APF are, Ms Nona said.
‘And when pharmacists did use them, they found the information provided was invaluable.’
‘[But] a lot of the challenges stemmed from lack of time and, in some cases, a lack of up-to-date knowledge. We have so many things to do, and we need more time to do everything and to keep ourselves up to date.’
For Ms Nona, the solution lies in supporting pharmacists to use guidelines confidently and consistently in real-world conditions.
Some pharmacists report to PSA that they will often bring up the APF digital on the screen in the consultation room in emergency contraception discussions, particularly in situations which are new or unfamiliar.
Delivering a critical intervention
The key to emergency contraception provision is recognising the stakes.
‘The whole picture of providing emergency contraception is to make sure we are preventing pregnancies when people don't want to get pregnant – whatever the reason may be,’ Ms Nona said. ‘That’s why they come to see a pharmacist – to ensure the person has the best possible chance of preventing an unintended pregnancy.’
When pharmacists are supported to provide full information and informed choice, patients respond accordingly.
The Australian Pharmaceutical Formulary and Handbook (APF) chapter on ‘Emergency Contraception’, provides essential guidance on:
|
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31305
[post_author] => 1925
[post_date] => 2026-02-08 10:38:16
[post_date_gmt] => 2026-02-07 23:38:16
[post_content] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
The range of professional services delivered by community pharmacists has expanded rapidly in recent years, from vaccination to UTI prescribing and beyond. As
these services increase in popularity, they are shifting from ancillary service to core business.
This widening scope is forcing community pharmacies to review how they conduct their business and the way front-of-house staff interact with patients.
No longer is dispensing prescriptions on a first come, first served basis sustainable. With adjustments to workflow, vaccinations and other booked services have been prioritised and run simultaneously, says Queensland-based prescribing pharmacist Kate Gunthorpe MPS.
‘We are moving away from the mindset that dispensing always comes first. We need to triage effectively and manage expectations, so every patient feels seen and cared for,’ she says.
And it isn’t just about sequential processes. Workflow changes also require a shift of communication approaches and pre-existing mindsets around professional service provision.
‘The biggest pitfall I’ve discovered is apologising for charging or determining that the consultation wasn’t worth charging for,’ Ms Gunthorpe says. ‘That instantly undermines the service’s value. Every consultation, whether the outcome is a prescription, advice or reassurance, involves clinical reasoning, professional judgement and patient care.’
So, how should the profession move forward? The PSA’s foundation documents are clear that all services must remain patient-centric.
That means redesigning workflows on the floor, developing new communication strategies for staff and providing additional training for pharmacy assistants to ensure a consistent, professional patient experience.
AP spoke with Ms Gunthorpe and pharmacy assistant Madison Low about adapting workflow to integrate services without disrupting dispensing. product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
Case 1 Kate Gunthorpe MPS
Pharmacist prescriber, Implementation and Change Specialist, TerryWhite Chemmart, Samford, Queensland
[caption id="attachment_31312" align="alignright" width="185"] Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Case 2 Madison Low
Retail manager, TerryWhite Chemmart, Arana Hills, Queensland
[caption id="attachment_31313" align="alignright" width="277"] Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31266
[post_author] => 11883
[post_date] => 2026-02-04 13:01:11
[post_date_gmt] => 2026-02-04 02:01:11
[post_content] => Case scenario
Greg, a 28-year-old man, comes into your pharmacy asking for a ‘strong minoxidil hair product’. He explains that his doctor recently diagnosed him with male pattern hair loss and suggested he try an over-the-counter treatment, with a follow-up review in 6 months. Greg has noticed gradual thinning at the temples over the past year but reports no sudden hair loss, scalp irritation or other medical issues. He has no known allergies, takes no medicines and has no chronic conditions.
Learning objectivesAfter reading this article, pharmacists should be able to:
|
Case scenario continuedAfter reviewing Greg’s history, you confirm there are no contraindications to minoxidil therapy. You explain the correct use of an over-the-counter foam formulation: applying to a dry scalp, taking care around the forehead and temples, waiting at least 1 hour before using other products and avoiding washing for 4 hours after application. You discuss the treatment timeline, reassuring Greg that initial shedding may increase but usually settles, and that it can take 3–4 months of consistent use before improvement is noticeable. You also emphasise the importance of follow-up with his doctor in 6 months and suggest simple supportive measures, such as protecting the scalp from sun exposure. Throughout the conversation, you address Greg’s concerns, reinforce realistic expectations, and encourage adherence to achieve the best outcome.1,2,8 |
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31273
[post_author] => 11884
[post_date] => 2026-02-04 12:59:14
[post_date_gmt] => 2026-02-04 01:59:14
[post_content] => Case scenario
Alicia, 27, visits your pharmacy regularly for naproxen and heat patches to manage period pain. She confides that her pain has worsened over the past 2 years, radiates down her legs, interferes with work and affects intimacy.
Her periods are heavy, lasting around 9 days, and leave her feeling exhausted and sometimes even bedridden. Alicia has seen several GPs, who told her it was ‘normal for your age’. She says, ‘It feels like someone’s wringing out my insides – nothing helps much. Is this really normal?’
Learning objectivesAfter reading this article, pharmacists should be able to:
|
 References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
Case scenario continuedYou reassure Alicia that severe period pain is not something she has to accept and suggest tracking her symptoms with a menstrual diary and consulting a women’s health GP. You also provide advice on safe NSAID use and non-pharmacological strategies. Alicia returns 2 months later, now diagnosed with endometriosis and receiving hormonal therapy and pelvic physiotherapy. She continues to experience chronic pelvic pain and questions her medicines, so you organise a Home Medicines Review, identifying potential naproxen overuse and interactions with her sertraline, prompting treatment adjustments. You also recommend a local endometriosis support group, which Alicia joins, and she has since referred two friends with similar symptoms. Through ongoing support, she feels more empowered to manage her condition. |
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31334
[post_author] => 3410
[post_date] => 2026-02-11 14:25:30
[post_date_gmt] => 2026-02-11 03:25:30
[post_content] => Victoria recently announced ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
But last week, the Allan Government also unveiled a nation-first initiative. From September, an online emergency prescribing pathway will allow people with an existing ADHD diagnosis to obtain urgent repeat medicine through the Victorian Virtual Emergency Department (VVED).
AP explores what the new model involves, the safeguards that will be in place and what’s happening in other states and territories.
What’s the purpose of the service?
The telehealth service is designed to address growing concern about long specialist waitlists, escalating costs and the clinical risks associated with abrupt cessation of Schedule 8 ADHD medicines – which can lead to symptom rebound, functional impairment and significant distress.
A Department of Health spokesperson told AP that the service would provide a targeted safety net, rather than a substitute for established care arrangements.
‘The Victorian Virtual Emergency Department will offer a safe way for Victorians with an existing ADHD diagnosis to refill an urgent prescription for ADHD medication,’ the spokesperson said.
How will urgent ADHD ‘repeats’ be issued?
Clinicians working within the VVED will verify the patient’s current medicine and dosage before issuing a prescription, the spokesperson said.
Scripts will be sent directly to the patient’s local pharmacy, with patients advised of the closest pharmacy in operation at the time of prescribing.
The Department of Health emphasised that the pathway will not replace routine ADHD management.
‘This is for emergency situations only and will not replace the important ongoing treatment and relationship between a patient and their clinician,’ the spokesperson said.
Who will be eligible?
The Victorian model allows adults and children aged 6 and over with an existing ADHD diagnosis to access the service who cannot secure a timely appointment with their usual clinician.
The service will be limited to people who are already prescribed ADHD medicines.
The VVED will not initiate ADHD treatment, alter dosages or provide ongoing prescribing.
What are the safeguards?
Existing regulatory requirements and clinical guidelines for ADHD medicines will remain fully in place under the VVED pathway.
‘The clinicians at the VVED, including paediatricians and psychiatrists, are highly experienced and highly skilled,’ the spokesperson said. ‘They will prescribe the medication within their existing scope of practice and clinical operations.’
Mandatory use of SafeScript for Schedule 8 medicines will continue to operate as a core safeguard. This ensures prescribers and pharmacists can monitor dispensing histories and reduces the risk of patients obtaining excessive prescriptions from multiple clinicians.
The Department has stressed that responsibility for ongoing ADHD management remains with the patient’s regular clinician, with the VVED acting solely as a one-off support mechanism that complements broader reforms aimed at expanding GP involvement in ADHD care.
And rather than providing a script to the patient, the script will be sent directly to their local pharmacy. When issuing a script, the VVED advises the patient of the closest pharmacy in operation.
Part of a broader national shift
Victoria’s online emergency model sits within a wider national trend to rebalance ADHD care away from exclusive reliance on specialist services.
Since 1 December 2025, ‘specialist GPs' in Queensland have been able to initiate, modify and continue stimulant treatment for adults with ADHD under updated Queensland Health guidance.
Today (11 February), ACT Health issued an announcement on ADHD prescribing, with GPs who have completed approved training now able to continue prescribing ADHD medicines for eligible patients without requiring repeated reviews from a psychiatrist, paediatrician or neurologist.
And other jurisdictions have since followed suit, including New South Wales, Western Australia and South Australia – which are set to roll out similar reforms this year.
Across Australia, governments are seeking to reduce wait times, lower out-of-pocket costs and embed ADHD care more firmly within primary care, while maintaining strong oversight of Schedule 8 stimulants such as methylphenidate, dexamfetamine and lisdexamfetamine via authorised prescribing schemes and real-time prescription monitoring checks. Non-stimulant ADHD medicines remain Schedule 4 and continue to be prescribed under existing arrangements.
For more information, complete the PSA online module: ADHD explained.
[post_title] => What pharmacists need to know about emergency prescribing for ADHD
[post_excerpt] => Victoria is the latest state to announce ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => what-pharmacists-need-to-know-about-emergency-adhd-prescribing
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-11 15:45:52
[post_modified_gmt] => 2026-02-11 04:45:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31334
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => What pharmacists need to know about emergency prescribing for ADHD
[title] => What pharmacists need to know about emergency prescribing for ADHD
[href] => https://www.australianpharmacist.com.au/what-pharmacists-need-to-know-about-emergency-adhd-prescribing/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31336
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31316
[post_author] => 3410
[post_date] => 2026-02-09 12:57:25
[post_date_gmt] => 2026-02-09 01:57:25
[post_content] => The gap between evidence and everyday practice is impacting patient access to emergency contraception.
Community pharmacies are often the first – and sometimes only – point of access for emergency contraception in Australia.
But a new qualitative study found there are still persistent gaps between what the guidelines say and what happens in practice.
Ruth Nona, pharmacist and researcher at James Cook University, who interviewed community pharmacists about providing emergency contraception services, describes a workforce that is broadly willing to help, but not always set up to deliver consistently equitable care.
1. Not recommending ulipristal as first-line
One of the most significant gaps identified in the study was the tendency for pharmacists to default to levonorgestrel, despite guidance in the Australian Pharmaceutical Formulary and Handbook (APF) that ulipristal acetate is generally considered more effective than levonorgestrel and can be used up to 120 hours after unprotected intercourse.
‘Habit definitely plays a role,’ Ms Nona said. ‘Some pharmacists felt more comfortable and confident supplying levonorgestrel. For example, if someone requested emergency contraception within 24 hours, pharmacists felt levonorgestrel was acceptable within that timeframe, without fully considering efficacy.’
[caption id="attachment_31329" align="alignright" width="250"] Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
‘If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information.'
Ruth nona
2. Uncertainty when responding to third-party requests
Pharmacists reported particular uncertainty when providing emergency contraception to third parties, with many wary about consent and unsure whether they could provide it to someone who wasn’t the patient.
‘In most cases, they would follow up and ask to contact the patient directly,’ Ms Nona said. ‘It wasn’t that they didn’t want to provide it – they just felt unsure and they wanted to make sure that the patient had consented.’
While in many cases, speaking to the intended person directly can help provide key information and counselling, in circumstances where this is not possible, it is usually possible to determine the medicine is safe and therapeutically appropriate, and supply in a manner consistent with APF guidance and legal obligations.
3. Uncertainty providing emergency contraception to adolescents
Similarly, pharmacists were ‘uncomfortable’ providing the service to adolescents.
This uncertainty often centred on fear of making the wrong decision or facing professional consequences.
‘They still wanted to provide the service, but it was more that internal question of, “Should I do this? Am I going to get in trouble?”’
There are no jurisdictions where there’s a legal restriction from supplying emergency contraception to minors. The APF guidance helps pharmacists navigate Gillick competency and consent in adolescents, ensuring they demonstrate sufficient maturity and understanding to provide informed consent.
Ms Nona emphasised that the issue was confidence, not capability.
‘It’s about being familiar with the guidelines, making sure we’re as up to date as possible and realising that it’s okay for us to provide these services, provided the adolescent is [assessed as Gillick competent] mentally mature and safe,’ she said.
The APF references Gillick competency provided the adolescent is [assessed as Gillick competent (demonstrating sufficient maturity and understanding to provide informed consent)]
4. Uncertainty for transgender people on gender affirming hormone therapy
While pharmacists were generally supportive towards transgender and gender-diverse patients, lack of familiarity with hormone therapy raised hesitation.
‘There are absolutely no interactions between emergency contraception and gender-affirming hormones’ Ms Nona said. ‘It really comes down to knowledge, which builds confidence, and being up to date to make sure the service we provide is timely and equitable.’
In some areas, pharmacists may frequently encounter transgender and gender- diverse patients requesting emergency contraception, while pharmacists in other areas do not.
‘That’s why it’s also about being prepared. You never know when that situation might arise,’ she added.
Should pharmacists feel unsure during these consultations, pharmacists can and should engage with the APF.
‘Pharmacists did say that if that situation did occur with a transgender or gender- diverse person that they would be honest and say to them, “Do you mind if I consult my resources?”’
Another reason the APF is a mandatory text for all community pharmacists
Despite lack of guideline use, pharmacists acknowledged how essential guidelines
such as the APF are, Ms Nona said.
‘And when pharmacists did use them, they found the information provided was invaluable.’
‘[But] a lot of the challenges stemmed from lack of time and, in some cases, a lack of up-to-date knowledge. We have so many things to do, and we need more time to do everything and to keep ourselves up to date.’
For Ms Nona, the solution lies in supporting pharmacists to use guidelines confidently and consistently in real-world conditions.
Some pharmacists report to PSA that they will often bring up the APF digital on the screen in the consultation room in emergency contraception discussions, particularly in situations which are new or unfamiliar.
Delivering a critical intervention
The key to emergency contraception provision is recognising the stakes.
‘The whole picture of providing emergency contraception is to make sure we are preventing pregnancies when people don't want to get pregnant – whatever the reason may be,’ Ms Nona said. ‘That’s why they come to see a pharmacist – to ensure the person has the best possible chance of preventing an unintended pregnancy.’
When pharmacists are supported to provide full information and informed choice, patients respond accordingly.
The Australian Pharmaceutical Formulary and Handbook (APF) chapter on ‘Emergency Contraception’, provides essential guidance on:
|
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31305
[post_author] => 1925
[post_date] => 2026-02-08 10:38:16
[post_date_gmt] => 2026-02-07 23:38:16
[post_content] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
The range of professional services delivered by community pharmacists has expanded rapidly in recent years, from vaccination to UTI prescribing and beyond. As
these services increase in popularity, they are shifting from ancillary service to core business.
This widening scope is forcing community pharmacies to review how they conduct their business and the way front-of-house staff interact with patients.
No longer is dispensing prescriptions on a first come, first served basis sustainable. With adjustments to workflow, vaccinations and other booked services have been prioritised and run simultaneously, says Queensland-based prescribing pharmacist Kate Gunthorpe MPS.
‘We are moving away from the mindset that dispensing always comes first. We need to triage effectively and manage expectations, so every patient feels seen and cared for,’ she says.
And it isn’t just about sequential processes. Workflow changes also require a shift of communication approaches and pre-existing mindsets around professional service provision.
‘The biggest pitfall I’ve discovered is apologising for charging or determining that the consultation wasn’t worth charging for,’ Ms Gunthorpe says. ‘That instantly undermines the service’s value. Every consultation, whether the outcome is a prescription, advice or reassurance, involves clinical reasoning, professional judgement and patient care.’
So, how should the profession move forward? The PSA’s foundation documents are clear that all services must remain patient-centric.
That means redesigning workflows on the floor, developing new communication strategies for staff and providing additional training for pharmacy assistants to ensure a consistent, professional patient experience.
AP spoke with Ms Gunthorpe and pharmacy assistant Madison Low about adapting workflow to integrate services without disrupting dispensing. product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
Case 1 Kate Gunthorpe MPS
Pharmacist prescriber, Implementation and Change Specialist, TerryWhite Chemmart, Samford, Queensland
[caption id="attachment_31312" align="alignright" width="185"] Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Case 2 Madison Low
Retail manager, TerryWhite Chemmart, Arana Hills, Queensland
[caption id="attachment_31313" align="alignright" width="277"] Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31266
[post_author] => 11883
[post_date] => 2026-02-04 13:01:11
[post_date_gmt] => 2026-02-04 02:01:11
[post_content] => Case scenario
Greg, a 28-year-old man, comes into your pharmacy asking for a ‘strong minoxidil hair product’. He explains that his doctor recently diagnosed him with male pattern hair loss and suggested he try an over-the-counter treatment, with a follow-up review in 6 months. Greg has noticed gradual thinning at the temples over the past year but reports no sudden hair loss, scalp irritation or other medical issues. He has no known allergies, takes no medicines and has no chronic conditions.
Learning objectivesAfter reading this article, pharmacists should be able to:
|
Case scenario continuedAfter reviewing Greg’s history, you confirm there are no contraindications to minoxidil therapy. You explain the correct use of an over-the-counter foam formulation: applying to a dry scalp, taking care around the forehead and temples, waiting at least 1 hour before using other products and avoiding washing for 4 hours after application. You discuss the treatment timeline, reassuring Greg that initial shedding may increase but usually settles, and that it can take 3–4 months of consistent use before improvement is noticeable. You also emphasise the importance of follow-up with his doctor in 6 months and suggest simple supportive measures, such as protecting the scalp from sun exposure. Throughout the conversation, you address Greg’s concerns, reinforce realistic expectations, and encourage adherence to achieve the best outcome.1,2,8 |
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31273
[post_author] => 11884
[post_date] => 2026-02-04 12:59:14
[post_date_gmt] => 2026-02-04 01:59:14
[post_content] => Case scenario
Alicia, 27, visits your pharmacy regularly for naproxen and heat patches to manage period pain. She confides that her pain has worsened over the past 2 years, radiates down her legs, interferes with work and affects intimacy.
Her periods are heavy, lasting around 9 days, and leave her feeling exhausted and sometimes even bedridden. Alicia has seen several GPs, who told her it was ‘normal for your age’. She says, ‘It feels like someone’s wringing out my insides – nothing helps much. Is this really normal?’
Learning objectivesAfter reading this article, pharmacists should be able to:
|
 References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
Case scenario continuedYou reassure Alicia that severe period pain is not something she has to accept and suggest tracking her symptoms with a menstrual diary and consulting a women’s health GP. You also provide advice on safe NSAID use and non-pharmacological strategies. Alicia returns 2 months later, now diagnosed with endometriosis and receiving hormonal therapy and pelvic physiotherapy. She continues to experience chronic pelvic pain and questions her medicines, so you organise a Home Medicines Review, identifying potential naproxen overuse and interactions with her sertraline, prompting treatment adjustments. You also recommend a local endometriosis support group, which Alicia joins, and she has since referred two friends with similar symptoms. Through ongoing support, she feels more empowered to manage her condition. |
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31334
[post_author] => 3410
[post_date] => 2026-02-11 14:25:30
[post_date_gmt] => 2026-02-11 03:25:30
[post_content] => Victoria recently announced ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
But last week, the Allan Government also unveiled a nation-first initiative. From September, an online emergency prescribing pathway will allow people with an existing ADHD diagnosis to obtain urgent repeat medicine through the Victorian Virtual Emergency Department (VVED).
AP explores what the new model involves, the safeguards that will be in place and what’s happening in other states and territories.
What’s the purpose of the service?
The telehealth service is designed to address growing concern about long specialist waitlists, escalating costs and the clinical risks associated with abrupt cessation of Schedule 8 ADHD medicines – which can lead to symptom rebound, functional impairment and significant distress.
A Department of Health spokesperson told AP that the service would provide a targeted safety net, rather than a substitute for established care arrangements.
‘The Victorian Virtual Emergency Department will offer a safe way for Victorians with an existing ADHD diagnosis to refill an urgent prescription for ADHD medication,’ the spokesperson said.
How will urgent ADHD ‘repeats’ be issued?
Clinicians working within the VVED will verify the patient’s current medicine and dosage before issuing a prescription, the spokesperson said.
Scripts will be sent directly to the patient’s local pharmacy, with patients advised of the closest pharmacy in operation at the time of prescribing.
The Department of Health emphasised that the pathway will not replace routine ADHD management.
‘This is for emergency situations only and will not replace the important ongoing treatment and relationship between a patient and their clinician,’ the spokesperson said.
Who will be eligible?
The Victorian model allows adults and children aged 6 and over with an existing ADHD diagnosis to access the service who cannot secure a timely appointment with their usual clinician.
The service will be limited to people who are already prescribed ADHD medicines.
The VVED will not initiate ADHD treatment, alter dosages or provide ongoing prescribing.
What are the safeguards?
Existing regulatory requirements and clinical guidelines for ADHD medicines will remain fully in place under the VVED pathway.
‘The clinicians at the VVED, including paediatricians and psychiatrists, are highly experienced and highly skilled,’ the spokesperson said. ‘They will prescribe the medication within their existing scope of practice and clinical operations.’
Mandatory use of SafeScript for Schedule 8 medicines will continue to operate as a core safeguard. This ensures prescribers and pharmacists can monitor dispensing histories and reduces the risk of patients obtaining excessive prescriptions from multiple clinicians.
The Department has stressed that responsibility for ongoing ADHD management remains with the patient’s regular clinician, with the VVED acting solely as a one-off support mechanism that complements broader reforms aimed at expanding GP involvement in ADHD care.
And rather than providing a script to the patient, the script will be sent directly to their local pharmacy. When issuing a script, the VVED advises the patient of the closest pharmacy in operation.
Part of a broader national shift
Victoria’s online emergency model sits within a wider national trend to rebalance ADHD care away from exclusive reliance on specialist services.
Since 1 December 2025, ‘specialist GPs' in Queensland have been able to initiate, modify and continue stimulant treatment for adults with ADHD under updated Queensland Health guidance.
Today (11 February), ACT Health issued an announcement on ADHD prescribing, with GPs who have completed approved training now able to continue prescribing ADHD medicines for eligible patients without requiring repeated reviews from a psychiatrist, paediatrician or neurologist.
And other jurisdictions have since followed suit, including New South Wales, Western Australia and South Australia – which are set to roll out similar reforms this year.
Across Australia, governments are seeking to reduce wait times, lower out-of-pocket costs and embed ADHD care more firmly within primary care, while maintaining strong oversight of Schedule 8 stimulants such as methylphenidate, dexamfetamine and lisdexamfetamine via authorised prescribing schemes and real-time prescription monitoring checks. Non-stimulant ADHD medicines remain Schedule 4 and continue to be prescribed under existing arrangements.
For more information, complete the PSA online module: ADHD explained.
[post_title] => What pharmacists need to know about emergency prescribing for ADHD
[post_excerpt] => Victoria is the latest state to announce ADHD reforms, with ‘specialist GPs’ now able to continue ADHD prescriptions for existing patients.
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => what-pharmacists-need-to-know-about-emergency-adhd-prescribing
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-11 15:45:52
[post_modified_gmt] => 2026-02-11 04:45:52
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31334
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => What pharmacists need to know about emergency prescribing for ADHD
[title] => What pharmacists need to know about emergency prescribing for ADHD
[href] => https://www.australianpharmacist.com.au/what-pharmacists-need-to-know-about-emergency-adhd-prescribing/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31336
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31316
[post_author] => 3410
[post_date] => 2026-02-09 12:57:25
[post_date_gmt] => 2026-02-09 01:57:25
[post_content] => The gap between evidence and everyday practice is impacting patient access to emergency contraception.
Community pharmacies are often the first – and sometimes only – point of access for emergency contraception in Australia.
But a new qualitative study found there are still persistent gaps between what the guidelines say and what happens in practice.
Ruth Nona, pharmacist and researcher at James Cook University, who interviewed community pharmacists about providing emergency contraception services, describes a workforce that is broadly willing to help, but not always set up to deliver consistently equitable care.
1. Not recommending ulipristal as first-line
One of the most significant gaps identified in the study was the tendency for pharmacists to default to levonorgestrel, despite guidance in the Australian Pharmaceutical Formulary and Handbook (APF) that ulipristal acetate is generally considered more effective than levonorgestrel and can be used up to 120 hours after unprotected intercourse.
‘Habit definitely plays a role,’ Ms Nona said. ‘Some pharmacists felt more comfortable and confident supplying levonorgestrel. For example, if someone requested emergency contraception within 24 hours, pharmacists felt levonorgestrel was acceptable within that timeframe, without fully considering efficacy.’
[caption id="attachment_31329" align="alignright" width="250"] Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
Ruth Nona[/caption]
In some pharmacies, price differences influenced whether ulipristal acetate was presented as an option.
‘In certain socio-economic areas, cost was a driver,’ Ms Nona said. ‘Cost considerations were also sometimes linked to younger people coming in and asking whether there was a cheaper option.’
Sometimes, levonorgestrel was the only medicine on hand.
‘There was research that came out showing that some pharmacies still did not stock ulipristal acetate,’ she said. ‘It’s been an ongoing issue, and something that really needs to be addressed.’
However, patients need to be able to make an informed decision about which medicine to take.
‘As stated in the guidelines, it’s about making sure all patients are given the information they need to make an informed and equitable choice. If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information,’ Ms Nona said.
‘When pharmacists did give the full picture and explained the differences, more often than not the patient chose ulipristal acetate, even though it cost more.’
‘If the patient wants a particular option, that’s fine, but they need to be able to make that decision with the full information.'
Ruth nona
2. Uncertainty when responding to third-party requests
Pharmacists reported particular uncertainty when providing emergency contraception to third parties, with many wary about consent and unsure whether they could provide it to someone who wasn’t the patient.
‘In most cases, they would follow up and ask to contact the patient directly,’ Ms Nona said. ‘It wasn’t that they didn’t want to provide it – they just felt unsure and they wanted to make sure that the patient had consented.’
While in many cases, speaking to the intended person directly can help provide key information and counselling, in circumstances where this is not possible, it is usually possible to determine the medicine is safe and therapeutically appropriate, and supply in a manner consistent with APF guidance and legal obligations.
3. Uncertainty providing emergency contraception to adolescents
Similarly, pharmacists were ‘uncomfortable’ providing the service to adolescents.
This uncertainty often centred on fear of making the wrong decision or facing professional consequences.
‘They still wanted to provide the service, but it was more that internal question of, “Should I do this? Am I going to get in trouble?”’
There are no jurisdictions where there’s a legal restriction from supplying emergency contraception to minors. The APF guidance helps pharmacists navigate Gillick competency and consent in adolescents, ensuring they demonstrate sufficient maturity and understanding to provide informed consent.
Ms Nona emphasised that the issue was confidence, not capability.
‘It’s about being familiar with the guidelines, making sure we’re as up to date as possible and realising that it’s okay for us to provide these services, provided the adolescent is [assessed as Gillick competent] mentally mature and safe,’ she said.
The APF references Gillick competency provided the adolescent is [assessed as Gillick competent (demonstrating sufficient maturity and understanding to provide informed consent)]
4. Uncertainty for transgender people on gender affirming hormone therapy
While pharmacists were generally supportive towards transgender and gender-diverse patients, lack of familiarity with hormone therapy raised hesitation.
‘There are absolutely no interactions between emergency contraception and gender-affirming hormones’ Ms Nona said. ‘It really comes down to knowledge, which builds confidence, and being up to date to make sure the service we provide is timely and equitable.’
In some areas, pharmacists may frequently encounter transgender and gender- diverse patients requesting emergency contraception, while pharmacists in other areas do not.
‘That’s why it’s also about being prepared. You never know when that situation might arise,’ she added.
Should pharmacists feel unsure during these consultations, pharmacists can and should engage with the APF.
‘Pharmacists did say that if that situation did occur with a transgender or gender- diverse person that they would be honest and say to them, “Do you mind if I consult my resources?”’
Another reason the APF is a mandatory text for all community pharmacists
Despite lack of guideline use, pharmacists acknowledged how essential guidelines
such as the APF are, Ms Nona said.
‘And when pharmacists did use them, they found the information provided was invaluable.’
‘[But] a lot of the challenges stemmed from lack of time and, in some cases, a lack of up-to-date knowledge. We have so many things to do, and we need more time to do everything and to keep ourselves up to date.’
For Ms Nona, the solution lies in supporting pharmacists to use guidelines confidently and consistently in real-world conditions.
Some pharmacists report to PSA that they will often bring up the APF digital on the screen in the consultation room in emergency contraception discussions, particularly in situations which are new or unfamiliar.
Delivering a critical intervention
The key to emergency contraception provision is recognising the stakes.
‘The whole picture of providing emergency contraception is to make sure we are preventing pregnancies when people don't want to get pregnant – whatever the reason may be,’ Ms Nona said. ‘That’s why they come to see a pharmacist – to ensure the person has the best possible chance of preventing an unintended pregnancy.’
When pharmacists are supported to provide full information and informed choice, patients respond accordingly.
The Australian Pharmaceutical Formulary and Handbook (APF) chapter on ‘Emergency Contraception’, provides essential guidance on:
|
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31305
[post_author] => 1925
[post_date] => 2026-02-08 10:38:16
[post_date_gmt] => 2026-02-07 23:38:16
[post_content] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
The range of professional services delivered by community pharmacists has expanded rapidly in recent years, from vaccination to UTI prescribing and beyond. As
these services increase in popularity, they are shifting from ancillary service to core business.
This widening scope is forcing community pharmacies to review how they conduct their business and the way front-of-house staff interact with patients.
No longer is dispensing prescriptions on a first come, first served basis sustainable. With adjustments to workflow, vaccinations and other booked services have been prioritised and run simultaneously, says Queensland-based prescribing pharmacist Kate Gunthorpe MPS.
‘We are moving away from the mindset that dispensing always comes first. We need to triage effectively and manage expectations, so every patient feels seen and cared for,’ she says.
And it isn’t just about sequential processes. Workflow changes also require a shift of communication approaches and pre-existing mindsets around professional service provision.
‘The biggest pitfall I’ve discovered is apologising for charging or determining that the consultation wasn’t worth charging for,’ Ms Gunthorpe says. ‘That instantly undermines the service’s value. Every consultation, whether the outcome is a prescription, advice or reassurance, involves clinical reasoning, professional judgement and patient care.’
So, how should the profession move forward? The PSA’s foundation documents are clear that all services must remain patient-centric.
That means redesigning workflows on the floor, developing new communication strategies for staff and providing additional training for pharmacy assistants to ensure a consistent, professional patient experience.
AP spoke with Ms Gunthorpe and pharmacy assistant Madison Low about adapting workflow to integrate services without disrupting dispensing. product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
Case 1 Kate Gunthorpe MPS
Pharmacist prescriber, Implementation and Change Specialist, TerryWhite Chemmart, Samford, Queensland
[caption id="attachment_31312" align="alignright" width="185"] Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Kate Gunthorpe MPS[/caption]
Our team started by mapping our busiest times to understand where bottlenecks occurred. We then built clear workflows – for example, using a booking system for consultations where possible, and ensuring at least one pharmacist remained consult-focused during every day.
We trained our assistants to triage appropriately and use consistent language, such as ‘the pharmacist will see you shortly for your consultation’, which helped the process feel deliberate rather than disruptive. Once the team understood that consultations were core services, not interruptions, the process flowed more smoothly.
Patients often expect a prescription outcome from a consultation, so I changed the framework, ensuring the consultation became a clinical one, not a product or medicines transaction. I now explain that I will assess their presentation, then create a unique shared management plan for them – which may or may not involve a prescription. Setting that expectation upfront helps enormously.
When we changed our front-of-shop language, patients stopped viewing consultations as waiting in a queue. That one shift in language lifted the professionalism of the whole process; patients were more patient, staff felt more confident explaining the service, and we saw an improvement in how people valued the pharmacist’s time.
One thing I would advise other pharmacists about charging appropriately for their time, even when the consultation doesn’t end with a script, is to start valuing their expertise. The consultation is the service, not the outcome. We’re expertly trained to assess, diagnose and provide evidence-based care. That deserves to be remunerated. Once pharmacists stop apologising and start consistently charging for their time and expertise, patients begin to respect that boundary too.
I find it is better to be transparent and consistent with pricing. I explain what’s included in the consultation, so patients understand what they’re paying for. Most importantly, I believe in it myself. If you hesitate to charge, your team and patients will pick up on that.
The work floor also needs redesigning to normalise consulting services as part of everyday care. It starts with the physical layout and staffing models. Pharmacies should expect consultations to happen and allocate dedicated private spaces, clear booking systems and enough pharmacist coverage, so that one can focus on clinical care, while others oversee dispensing and other services. Culturally, it’s all been about mindset: we stopped treating services as extra and started treating them as central to what we do.
That meant redistributing roles, upskilling support staff with more autonomy to triage and manage appointments, and introducing appointment blocks.
The effect of all these new processes has resulted in major change. Our pharmacy assistants are our front line, and their language is setting the tone for the entire patient experience. We have invested time in scripting and role-playing, so that the assistants feel comfortable discussing new services. The staff have learned to understand what each service involves, how long it typically takes, and when to book or triage patients.
Scripts no longer automatically take priority over walk-up service requests. They are both core services. Pharmacies are healthcare hubs where patients can expect to have a prescription filled but also be able to discuss their health concerns.
Patients also value honesty and clarity. If a medication is not appropriate, explaining why builds understanding and trust – especially when you provide alternative options or safety net advice.
Because pharmacy staff use consistent, confident language and understand the workflow, everything runs much more smoothly. It has also empowered the staff to take pride in being responsible for a part of the patient care process, not just the retail side.
When staff describe services as core health care, not as ‘extras’ or ‘add-ons’, patients have started to see the pharmacist as a clinician involved in their primary care.
It’s a subtle but powerful mindset shift that’s transforming how the pharmacy is perceived.
Case 2 Madison Low
Retail manager, TerryWhite Chemmart, Arana Hills, Queensland
[caption id="attachment_31313" align="alignright" width="277"] Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
Madison Low[/caption]
Since we started offering services like UTI consultations and vaccinations, my role has expanded significantly. We no longer just provide products; we’re delivering a more complete healthcare solution.
A person recently came to the counter, visibly frustrated because they had symptoms of a urinary tract infection but couldn’t get in to see their doctor. They were holding a box of Ural.
Rather than just selling them the product, I suggested they talk to the pharmacist, assuring them that in many cases the pharmacist can provide a full treatment without needing a doctor’s visit.
I asked a few questions about their symptoms, then checked with the pharmacist to confirm a consultation was possible immediately. It was, and not long afterwards the patient went away happy.
Asthma management is one of the most common chronic conditions we see. Many patients believe they understand how to manage the condition, especially because they can access inhalers over the counter. But often that’s not the case. One of my roles is to let patients know there may be better solutions. Our pharmacists can review their current treatment and provide an improved management plan.
Since becoming more mindful of the language I use with patients, I’ve noticed a positive change in how they respond to me.
By communicating in a more empathetic and approachable way, I’ve found patients are more comfortable asking questions and discussing their concerns. This has made it easier to identify when a patient might benefit from a review with one of our pharmacists.
This change in language has also strengthened trust between patients and the pharmacy team. Patients seem more engaged and confident in the care they receive, and I feel more confident in my role as a link between them and our pharmacists.
The biggest challenge has been balancing our time – especially during busy periods like the flu season, when there are lots of vaccines to administer, prescriptions to dispense and consultations to organise. I’m proud of how our team works together to ensure our patients are looked after promptly and get the attention they need.
[post_title] => How expanded scope is redefining pharmacy practice
[post_excerpt] => Rapidly evolving scope of practice means that traditional community pharmacy workflows need review. What works (and what doesn’t)?
[post_status] => publish
[comment_status] => open
[ping_status] => open
[post_password] =>
[post_name] => how-expanded-scope-is-redefining-pharmacy-practice
[to_ping] =>
[pinged] =>
[post_modified] => 2026-02-09 14:54:19
[post_modified_gmt] => 2026-02-09 03:54:19
[post_content_filtered] =>
[post_parent] => 0
[guid] => https://www.australianpharmacist.com.au/?p=31305
[menu_order] => 0
[post_type] => post
[post_mime_type] =>
[comment_count] => 0
[filter] => raw
)
[title_attribute] => How expanded scope is redefining pharmacy practice
[title] => How expanded scope is redefining pharmacy practice
[href] => https://www.australianpharmacist.com.au/how-expanded-scope-is-redefining-pharmacy-practice/
[module_atts:td_module:private] => Array
(
)
[td_review:protected] => Array
(
)
[is_review:protected] =>
[post_thumb_id:protected] => 31310
[authorType] =>
)
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31266
[post_author] => 11883
[post_date] => 2026-02-04 13:01:11
[post_date_gmt] => 2026-02-04 02:01:11
[post_content] => Case scenario
Greg, a 28-year-old man, comes into your pharmacy asking for a ‘strong minoxidil hair product’. He explains that his doctor recently diagnosed him with male pattern hair loss and suggested he try an over-the-counter treatment, with a follow-up review in 6 months. Greg has noticed gradual thinning at the temples over the past year but reports no sudden hair loss, scalp irritation or other medical issues. He has no known allergies, takes no medicines and has no chronic conditions.
Learning objectivesAfter reading this article, pharmacists should be able to:
|
Case scenario continuedAfter reviewing Greg’s history, you confirm there are no contraindications to minoxidil therapy. You explain the correct use of an over-the-counter foam formulation: applying to a dry scalp, taking care around the forehead and temples, waiting at least 1 hour before using other products and avoiding washing for 4 hours after application. You discuss the treatment timeline, reassuring Greg that initial shedding may increase but usually settles, and that it can take 3–4 months of consistent use before improvement is noticeable. You also emphasise the importance of follow-up with his doctor in 6 months and suggest simple supportive measures, such as protecting the scalp from sun exposure. Throughout the conversation, you address Greg’s concerns, reinforce realistic expectations, and encourage adherence to achieve the best outcome.1,2,8 |
td_module_mega_menu Object
(
[post] => WP_Post Object
(
[ID] => 31273
[post_author] => 11884
[post_date] => 2026-02-04 12:59:14
[post_date_gmt] => 2026-02-04 01:59:14
[post_content] => Case scenario
Alicia, 27, visits your pharmacy regularly for naproxen and heat patches to manage period pain. She confides that her pain has worsened over the past 2 years, radiates down her legs, interferes with work and affects intimacy.
Her periods are heavy, lasting around 9 days, and leave her feeling exhausted and sometimes even bedridden. Alicia has seen several GPs, who told her it was ‘normal for your age’. She says, ‘It feels like someone’s wringing out my insides – nothing helps much. Is this really normal?’
Learning objectivesAfter reading this article, pharmacists should be able to:
|
 References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
References: Therapeutic Guidelines1, Buggio et al12, Rossi13 Hornstein26, Vercellini27
Note: Bone mineral density typically recovers within two years of cessation of GnRH therapies.27
Drospirenone currently off-label for endometriosis in Australia.1
The addition of Ryeqo (relugolix, estradiol, norethisterone) (July 2022) and Visanne (dienogest) (December 2024) to the PBS expands accessible hormonal therapy options for endometriosis.29
Case scenario continuedYou reassure Alicia that severe period pain is not something she has to accept and suggest tracking her symptoms with a menstrual diary and consulting a women’s health GP. You also provide advice on safe NSAID use and non-pharmacological strategies. Alicia returns 2 months later, now diagnosed with endometriosis and receiving hormonal therapy and pelvic physiotherapy. She continues to experience chronic pelvic pain and questions her medicines, so you organise a Home Medicines Review, identifying potential naproxen overuse and interactions with her sertraline, prompting treatment adjustments. You also recommend a local endometriosis support group, which Alicia joins, and she has since referred two friends with similar symptoms. Through ongoing support, she feels more empowered to manage her condition. |
The social highlight of the annual PSA conference is the Gala Dinner, and this year certainly did not disappoint.
Get your weekly dose of the news and research you need to help advance your practice.
Protected by Google reCAPTCHA v3.

Australian Pharmacist is the official journal for Pharmaceutical Society of Australia Ltd.
