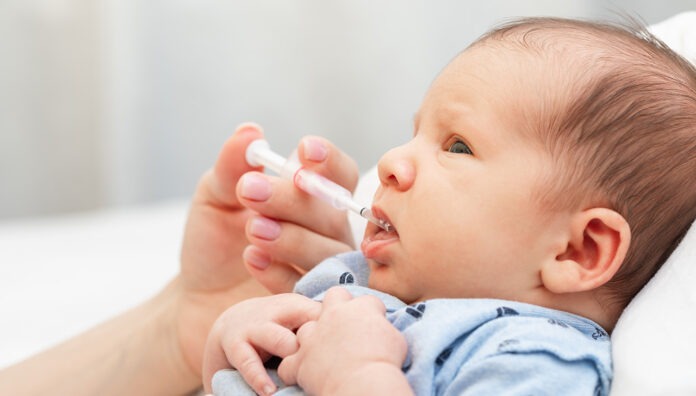
Early-life exposure to antibiotics can weaken the immune response to several routine vaccinations in infants, research has found.
Pharmacists may have had questions this week about antibiotic use in children following news articles highlighting the link between some antibiotics and vaccine efficacy. AP looks behind the headline to help pharmacists know how to respond
What’s driving this impairment?
Infants exposed to antibiotics in the neonatal period or first year of life had significantly lower antibody responses to vaccines including meningococcal ACWY and measles, mumps, rubella, found a study led by Australian experts.
The impact on antibody response is most likely due to disruptions in the development of gut bacteria.
‘We’ve known for some time that gut bacteria play an important role in shaping the immune system, but this study provides strong evidence that early life antibiotics can disrupt that process in a way that weakens vaccine responses,’ said Professor David Lynn, Professor of Systems Immunology at Flinders Health and Medical Research Institute, who co-led the study.
Infants who were treated with antibiotics had lower amounts of helpful gut bacteria at the time of vaccination, with Bifidobacteria particularly impacted. Children with this reduction in gut bacteria later showed weaker immune responses, indicated by reduced antibody levels at 6 and 14 months of age.
‘This suggests that these gut bacteria play a key role in helping the immune system respond optimally to vaccines,’ he said.
Interestingly, the study also found that babies whose mothers received antibiotics during labour did not have reduced vaccine responses.
‘This is an important distinction because it suggests that not all antibiotic exposure carries the same risks when it comes to the impact on the infant’s immune responses,’ Prof Lynn said. ‘[These findings] raise important questions about how we use antibiotics in newborns and what we can do to reduce any unintended consequences.’
What should pharmacists do?
Pharmacists should advise patients that routine vaccinations should not be postponed. At this stage, there have also not been changes to guidelines around antibiotic use. And antibiotics should also not be withheld when required, with infant infections often presenting as severe and requiring urgent treatment.
‘There’s usually a very good reason for giving the neonates those antibiotics, given that infections and sepsis in that critical early life period can be very serious,’ Prof Lynn told the Guardian.
Furthermore, infants treated with antibiotics still produce a sufficient immune response when vaccinated.
‘Around that 7-month time point, most of the infants are above what’s called the seroprotective threshold, so they will be expected to be protected against infection,’ Prof Lynn said.
‘What does seem to happen is that, over time, those responses wane a bit quicker in the infants that directly have antibiotics.’
The gut microbiome can also likely be repaired through the use of prebiotics and probiotics after antibiotic exposure, boosting impaired vaccine responses, found in an earlier preclinical study in mice led by Prof Lynn.
But antibiotics should continue to be used wisely.
‘In view of the importance of vaccination in maintaining health in society, this is yet another reason why antibiotics should be administered judiciously,’ said Associate Professor Peter Speck, from the College of Science and Engineering at Flinders University. ‘Antibiotic stewardship is clearly of great value, especially in the neonatal setting.’
What are the next steps?
While the findings are compelling, the authors acknowledge there are several limitations to the research . The study cohort was relatively small, and numbers in each antibiotic-exposed subgroup were even smaller.
Infants born by caesarean section or to mothers with a body mass index above 30 were excluded, so the results may not reflect the broader population seen in everyday practice. Immune responses were assessed with only limited functional assays and no detailed T cell analysis. Larger, more diverse studies will be needed to confirm and build on these early findings.
To that end, a human trial funded by the Women’s and Children’s Hospital Foundation is set to investigate if the infant immune response to vaccines can be improved by giving probiotics to babies treated with antibiotics in the first week of life.
‘This will provide evidence as to whether this simple probiotic intervention can support optimal immune responses to vaccination in early life, and we’ll also be able to identify the molecular mechanisms governing the differences in vaccine efficacy,’ Prof Lynn said.
‘Our findings could also be relevant to long-term child health, given prior associations between antibiotic exposure and an increased propensity to develop conditions such as allergies, asthma and obesity.’
Visit PSA’s Vaccination (Immunisation) Education Hub to access vaccination education and resources.




 Owner of Canberra's Capital Chemist Southlands Louise McLean MPS.[/caption]
Owner of Canberra's Capital Chemist Southlands Louise McLean MPS.[/caption]

 Supplied by CSL Seqirus[/caption]
Supplied by CSL Seqirus[/caption]


 Tahnee Simpson[/caption]
Tahnee Simpson[/caption]
 Nicolette Ellis MPS[/caption]
Nicolette Ellis MPS[/caption]