Real-time prescription monitoring (RTPM) is gaining momentum and now operates across large swathes of Australia. What does it mean for pharmacists?
Pharmacists in Victoria, the Australian Capital Territory and Tasmania have been using RTPM systems for some time now and Queensland pharmacists are due to join their ranks later this year.
Other parts of Australia might not be too far behind either, after a spokesperson for the Federal Health Minister told The Age that Greg Hunt had written to the remaining jurisdictions urging them to connect to a national monitoring system ‘as a matter of priority’.
‘The Commonwealth expects all other states and territories to integrate their systems by the end of this year, if not the middle of this year,’ Mr Hunt’s spokesman reportedly said in February 2020.1
So what can be learned from those already using RTPM?
Safescript in Victoria
Victoria’s RTPM solution, SafeScript, was introduced to the Western Victoria Primary Health Network in October 2018 and went statewide 6 months later.
On April, 1 it will become compulsory to check SafeScript prior to writing or dispensing a prescription for all high-risk medicines.
PSA Project Pharmacist Jarrod McMaugh, said the impact on pharmacists’ workflow had been minimal.
‘SafeScript has actually integrated very, very well. Workflow is not being disrupted, as long as you’re using an integrated dispensing software,’ he said.
Lessons
The SafeScript system uses three different colour-coded pop-ups – green, orange and red – which indicate whether a patient meets a criteria that could warrant further investigation.
Mr McMaugh said: ‘It’s important to remember the red flag is not the point when you make a decision not to dispense. It’s the point where you would say “okay we need to look further and have a conversation with the person”.’
When looking into a patient’s dispense history Mr McMaugh said it was not only important to look at what had been dispensed recently, but also what had not.
‘If a pharmacist has logged in earlier, decided not to dispense and just undid the script, SafeScript doesn’t register the decision not to dispense,’ he said.
‘It does, however, register that they logged in, which is a good clue.’
In order to send a clearer message to other pharmacists, McMaugh suggested processing the script and then cancelling it.
‘That cancellation will show up in the record and may lead to discussions the next time the patient presents to a pharmacist or a doctor,’ he said.
DORA in the ACT
The Australian Capital Territory introduced its RTPM system in March 2019. The system – DORA – taps into the territory’s existing Drugs and Poisons Information System and the Federal Government’s National Data Exchange (NDE). Using DORA remains voluntary for both pharmacists and prescribers and uptake is reportedly low: about 1 in 5 prescribers and 1 in 3 pharmacists use it.2
That said, many pharmacies are connected to a Prescription Exchange Service and registered for DORA which ensures prescription data is being automatically transferred to the NDE.
Lessons
PSA ACT Branch President Renae Beardmore said pharmacists who were using the system were noticing benefits.
‘They’ve said to me that you see information that you can’t unsee,’ she said.
‘One pharmacist said they thought a patient only came to them and they were very surprised to see the patient had been attending multiple other pharmacies. RTPM is about giving the pharmacist the fullest information possible to allow them to make a clinical decision to supply or not.’
Unlike SafeScript – which is fully automated – DORA involves dispensing information going through to operators who monitor data and provide feedback
to pharmacies.
While outcomes had been good, Ms Beardmore said it would be difficult for bigger jurisdictions to replicate the ACT’s approach to RTPM.
‘We only have 80-odd pharmacies in the ACT so being able to communicate with pharmacies in a fax stream straight away when there’s a problem is something that other jurisdictions don’t have,’ she said.
Benefits of RTPM
In Tasmania pharmacists have been using DORA to support decision-making around high-risk medicines since 2011.
There, the per capita death rate from prescription opioids has fallen from approximately 30% above the national average (between 2002 and 2006) to 27% below the national average (between 2012 and 2016).3
PSA Tasmania’s Ella van Tienen said RTPM was not something to be scared or apprehensive about.
‘Having access to this information is such a useful tool to add to your ability to make sound clinical decisions on the appropriateness of supply of drugs with the potential for abuse,’ she said.
More recently in Victoria, RTPM has helped identify suspected forgeries, said PSA Senior Pharmacist – Strategic Policy, Peter Guthrey.
‘Sometimes the absence of information in RTPM systems can be just as important as information you are seeing,’ he said.
‘When supplying a high-potency opioid, such as fentanyl patches, an empty patient history is unusual and is worth exploring further. This could be a clue to a forged or illegally altered prescription.’
Mr Guthrey said this example highlighted that while SafeScript was a useful tool, pharmacists needed to continue using their own judgement and engage with patients.
‘A red flag doesn’t mean you should not supply, just as a green tick doesn’t mean you should supply blindly. RTPM flags are risk-stratified tools to help you make informed medicine safety decisions for your patients,’ he said. ‘You should be exploring the patient’s health needs further to understand what is and is not safe medicine therapy and supply.’
A spokesperson for the Department of Health and Human Services Victoria told AP that latest data showed there had been a 25% reduction in the proportion of people taking high-risk doses of opioids in Victoria since SafeScript was rolled out statewide. They noted that more than 22,000 health professionals had registered to use SafeScript, including over 77% of pharmacists and 78% of GPs.
But while the benefits of RTPM across the country have been noteworthy, Ms Beardmore warned that RTPM was not a silver bullet.
‘RTPM is not the holy grail, it’s not the panacea. It’s important, but it is only part of the solution,’ she said.
FIGURE 2 – RTPM* across the country
| JURISDICTION | RTPM* | REVIEW |
|---|---|---|
| Tasmania | Yes | Tasmania’s RTPM system, the Drugs and Poisons Information System Online Remote Access (DORA), was made available to clinicians in 2011. Use of the DORA website by clinicians considering prescribing or supplying high-risk medicines remains voluntary. |
| Victoria | Yes | Victoria’s RTPM solution, SafeScript, was introduced to the Western Victoria Primary Health Network in October 2018. It went statewide six months later. From 1 April 2020 it will be compulsory to check SafeScript prior to writing or dispensing a prescription for all monitored medicines. |
| Australian Capital Territory | Yes | The ACT introduced DORA in March 2019. Using DORA remains voluntary for both pharmacists and prescribers. Uptake is reportedly low: about one in five prescribers and one in three pharmacists use it. |
| Queensland | No | The system will be known as QScript and the government said it would be ready for use in 2020. The pharmacy IT company behind SafeScript, Fred IT Group, signed an agreement with the Queensland government in October 2019 to develop an RTPM system. |
| South Australia | No | The South Australian government’s 2018-19 budget committed $4 million to implementing an RTPM system. |
| New South Wales | No | The NSW Government has allocated $1.35 million in seed funding to connect to a national RTPM monitoring system, a health department spokeswoman reportedly said. |
| Western Australia | No | The West Australian government is working with industry to transition to RTPM.** |
| Northern Territory | No |
*RTPM system in operation April 2020
** More information at: ww2.health.wa.gov.au/Articles/N_R/Prescription-monitoring-in-Western-Australia
Initiating difficult conversations
Whether pharmacists are operating in a jurisdiction with RTPM or not, it’s important that they initiate difficult conversations with patients if they’re concerned about dispensing medicine.
‘It’s not enough to say “no, I’m not dispensing”. You have to be part of the solution,’ Ms Beardmore said.
She encouraged pharmacists to approach difficult conversations with empathy.
‘As soon as you confront somebody, it’s natural human behaviour for them to be defensive,’ she said.
Mr McMaugh said his conversations often went something like this: ‘The type of medicine that you’re getting filled is part of a system that helps us see your total history for this medicine. And based on that history I’m concerned that it could be unsafe for you to have this medicine today.’
He said the topics pharmacists may want to explore during their conversations were:
- what has the patient been told about the medicine?
- how often are they using it compared to what has been prescribed?
- are they using more because their pain is not well controlled?
- is it possible that they’re sharing their medicine with others?
‘People will quite often open up when they realise that the reason you’re asking the questions is for their safety, rather than protecting the medicines,’ Mr McMaugh said.
Ms Van Tienen reassured pharmacists that the conversations became easier.
‘It’s always daunting initiating difficult conversations with people you’ve deemed supply of medicine is inappropriate for – but it does get easier,’ she said.
Supporting people with monitored medicines
After identifying what the underlying issue may be, pharmacists need to be ready to provide appropriate support, according to Mr Guthrey.
‘Where medicine safety problems are identified, pharmacists need to take appropriate action to improve patient safety,’ he said.
‘This may include working with the patient’s prescriber, delaying part or all of the prescribed medicine supply, recommending rescue medicines such as naloxone or linking into support services – but in all cases, having an open dialogue with your patient is essential.
‘What is often surprising is that brief counselling interventions – working through with a person on how their medicine works and how it aligns to their health needs – are extremely powerful in helping manage risks associated with monitored medicines.’
Mr McMaugh added that it was important to carefully tailor referrals to patient needs.
‘If you’ve got somebody with a pain condition that’s just poorly controlled, referring them to opioid substitution therapy is not going to be appropriate,’ he said.
‘Or somebody with endometriosis and chronic pain due to that probably wouldn’t benefit from a physiotherapist, whereas somebody with osteoarthritis might.
‘It’s a matter of knowing who to refer to and not assuming that every person presenting to you is going to have a substance use disorder.’
Mr Guthrey said pharmacists looking to provide patients with the very best support could look to a range of tools and resources, including PSA workshops on opioid management, training delivered by PHNs, Painaustralia resources,3 or the NPS Medicinewise chronic pain management hub.
References
- Tomazin F. Opioid crisis: Australian states told to act now or suffer a public health emergency. The Age. 2020. At: www.theage.com.au/national/opioid-crisis-australian-states-told-to-act-now-or-suffer-a-public-health-emergency-20200206-p53yeu.html
- Maddocks T. Accidental overdose victim’s daughter calls for mandatory scheme to stop dangerous drug sales. ABC News. 2020. At: www.abc.net.au/news/2020-02-13/overdose-victims-daughter-calls-for-wider-drug-monitoring/11959134
- Pain Australia. Health Professionals Resources. At: www.painaustralia.org.au/




 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.

 Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4
Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4  C – Comorbidity and risk factor management
C – Comorbidity and risk factor management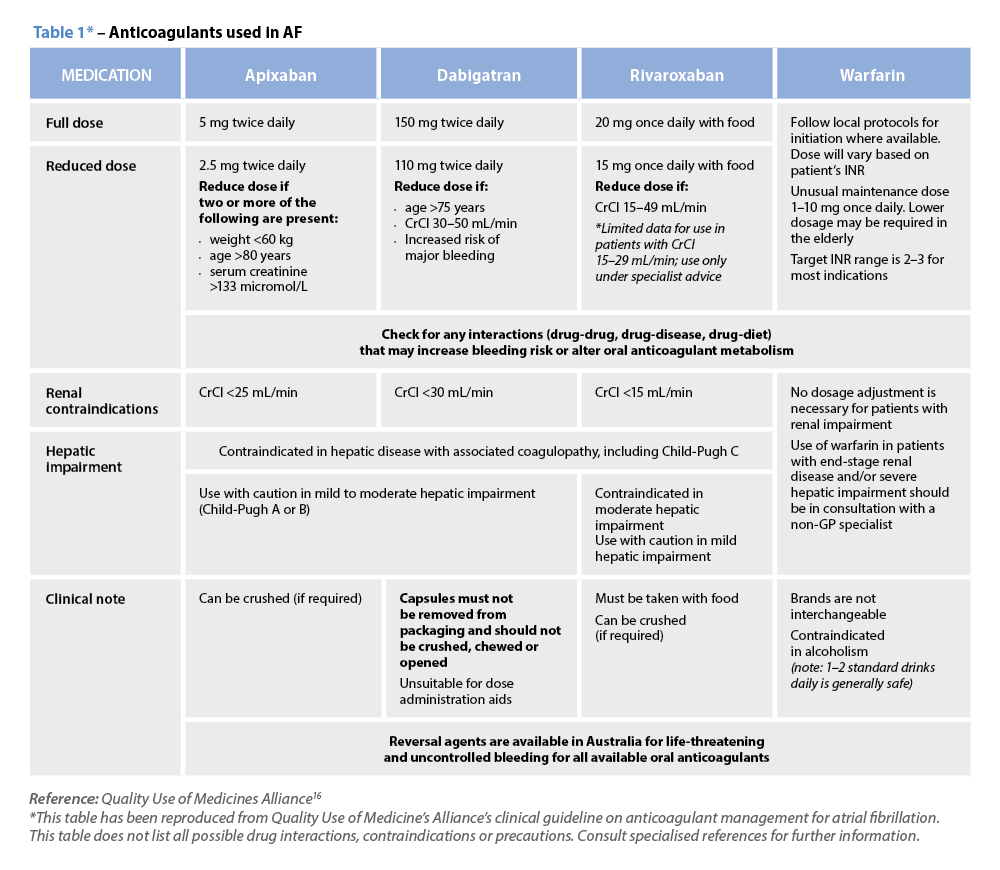 Warfarin
Warfarin




