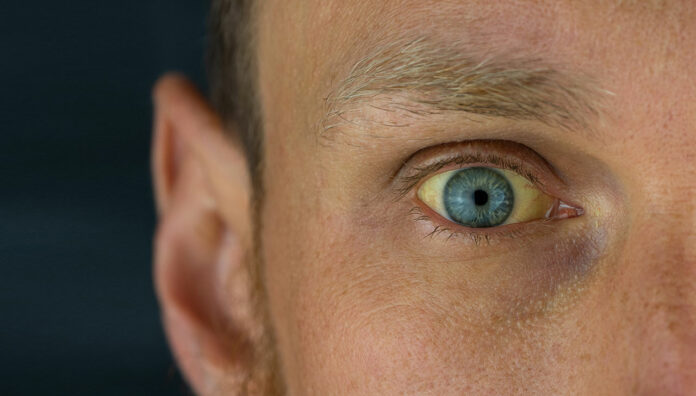
Thiazolidinediones may hold the key to developing a new class of anti-HBV medicines that will cure the chronic infection.
Statistics from 2018 estimate that 226,566 people (0.9% of the population) in Australia live with chronic hepatitis B, but only 68.1% of those living with the condition have ever been diagnosed.1 Unfortunately, of those living with chronic hepatitis B, only 22.1% are engaged in regular care (i.e. monitoring or antiviral therapy), and 9.3% are receiving antiviral therapy.1
Australia has set national targets to improve on these statistics, and has signed on to the World Health Organization global hepatitis elimination targets for 2030.1
Injury to the liver is caused by the immune system’s response and attempt to remove the hepatitis B virus (HBV), which results in 25% of people with chronic hepatitis B ‘developing cirrhosis which progresses to end-stage liver disease, and there is a 200-fold increase in risk of hepatocellular carcinoma’.2
While not all people living with chronic hepatitis B will require treatment (e.g. those experiencing immune tolerance or immune control), regular check-ups are important to help determine when treatment should be considered (e.g. during immune clearance or immune escape).3–5
The goal of therapy is not curative per se, as loss of the hepatitis B surface antigen (HBsAg) occurs in less than 1% of treated patients annually.5 Instead, the aim is to reduce/prolong the suppression of the HBV load to below detectable limits, to prevent or delay progression to cirrhosis, liver failure and hepatocellular carcinoma.4–6
Treatment is complex, so input from specialists (e.g. gastroenterologists or hepatologists) or other practitioners accredited to treat hepatitis B is important.5,7
Initial treatment options include the nucleos(t)ide analogues – tenofovir and entecavir, or peginterferon alfa-2a (‘inhibits viral replication and has immunomodulatory effects’).7 Tenofovir is ‘metabolised to the active tenofovir diphosphate, which inhibits viral polymerases and terminates the DNA chain after incorporation into viral DNA’,7 while entecavir ‘is a guanosine analogue, and entecavir triphosphate inhibits hepatitis B polymerase, preventing viral DNA synthesis’.7 The other nucleos(t)ide analogues – adefovir and lamivudine – are no longer recommended as initial treatment due to high rates of resistance.5,7
While tenofovir and entecavir are generally well tolerated and have long-term efficacy, it is uncommon for these agents to achieve undetectable HBV DNA levels – so lifelong therapy may be required.7 Peginterferon alfa-2a has shorter treatment courses but is also poorly tolerated, with variable responses, and relapse after completion of a peginterferon alfa-2a course is also more common compared to long-term nucleos(t)ide analogues.7 Therefore, the search is still on for other management options for hepatitis B, particularly one that can lead to a cure.
A class of medicine that has fallen out of favour as oral-antihypoglycaemics is the thiazolidinediones (or glitazones), and these have sparked interest during cell-based chemical screening studies.8 While this is not a class of medicine one would immediately associate with HBV, or any viral infection, recent work has highlighted that troglitazone impedes the entry of HBV into hepatocytes.8
Troglitazone was the first medicine in the class to the market around 1997, but was withdrawn soon after due to hepatotoxicity and deaths from liver failure.9 Many of the medicines in the class have also been withdrawn due to adverse effects, or have been replaced by newer oral-antihypoglycaemics that are safer and more effective.
The thiazolidinediones worked by ‘increasing insulin secretion or making the peripheral cells more sensitive to insulin’,10 ‘through agonist activity of peroxisome proliferator-activated receptor-γ (PPAR γ)’,8 ‘which regulates genes involved in lipid and glucose metabolism’.11
The work through a functional cell-based chemical screening indicated that troglitazone’s ability to impede the internalisation of the HBV was independent of its activity at PPAR γ.8 The ability to block the virus entry and uptake into cells is of significant interest as a new agent, as viral entry plays an important role in the start, spread and maintenance of infection.8
The interaction between the large surface protein (preS1 region) of HBV with the entry receptor sodium taurocholate cotransporting polypeptide (NTCP) is noted to be important for triggering internalisation, but it is not well understood how this occurs.8 The authors of the study reported NTCP to be markedly oligomerised in the presence of HBV preS1, but this oligomerisation was prevented by treatment with troglitazone, correlating with inhibition of viral internalisation activity.8 Structure activity relationship work also found the thiazolidinedione moiety important for the anti-HBV effects, though this was not the case for the comparatively smaller pioglitazone, which showed little to no effect on HBV infection.8
Much more work is undoubtedly required to understand the biological significance of NTCP oligomerisation, and specific molecules that can interfere with that process. While the existing thiazolidinediones may not be the answer right now, there is interest and investment in developing novel thiazolidinediones as anticancer medicines,8 and this work potentially assists in the development of a new class of anti-HBV medicines that may provide hope for a cure.
References
- Hepatitis Australia. Hepatitis Statistics Woden (ACT): Hepatitis Australia; 2020 At: www.hepatitisaustralia.com/hepatitis-statistics
- Paxton G, Clifford V. Hepatitis B Parkville (VIC): The Royal Children’s Hospital Melbourne; 2013. Updated 2020. At: www.rch.org.au/immigranthealth/clinical/Hepatitis_B/
- Australasian Society for HIV VHaSHM. Decision Making in Hepatitis B Sydney (NSW): ashm; 2021. At: www.ashm.org.au/resources/HBV-Resources-list/decision-making-in-hbv/
- Australasian Society for HIV VHaSHM. Hepatitis B and Primary Care Providers Sydney (NSW): ashm; 2012. At: www.ashm.org.au/resources/HBV-Resources-list/hepatitis-b-and-primary-care-providers/
- Expert Group for Liver Disorders. Therapeutic guidelines: Liver Disorders. Version 1 – Hepatitis B. Melbourne: Therapeutic Guidelines Limited; 2021.
- Hepatitis Australia. What is hepatitis B Woden (ACT): Hepatitis Australia; 2020. At: www.hepatitisaustralia.com/what-is-hepatitis-b
- Australian Medicines Handbook. Australian Medicines Handbook – Antivirals for hepatitis B: Australian Medicines Handbook Pty Ltd; 2021.
- Fukano K, Tsukuda S, Oshima M, et al. Troglitazone impedes the oligomerization of sodium taurocholate cotransporting polypeptide and entry of hepatitis B virus into hepatocytes. Front Microbiol 2019;9:3257.
- Shenfield GM. Have glitazones lost their sparkle? Aust Prescr 2008;31:58–9.
- NPS Medicinewise. Troglitazone. Aust Prescr 1999;22:147–51.
- Australian Medicines Handbook. Australian Medicines Handbook – Pioglitazone: Australian Medicines Handbook Pty Ltd; 2021.
Dr ESTHER LAU BPharm (Hons), PhD, GCResComm, GradCertAcadPrac, AACPA, MPS is A/Head of Discipline and Senior Lecturer in the Discipline of Pharmacy at the Queensland University of Technology (QUT).
Professor LISA NISSEN BPharm, PhD, FPS, FHKAPh, FSHPis the Head, School of Clinical Sciences, at QUT.



 John Jones MPS, pharmacist immuniser and owner of My Community Pharmacy Shortland in Newcastle, NSW[/caption]
John Jones MPS, pharmacist immuniser and owner of My Community Pharmacy Shortland in Newcastle, NSW[/caption]


 Debbie Rigby FPS explaining how to correctly use different inhaler devices[/caption]
Debbie Rigby FPS explaining how to correctly use different inhaler devices[/caption]




 Professor Sepehr Shakib[/caption]
Professor Sepehr Shakib[/caption]

 Lee McLennan MPS[/caption]
Lee McLennan MPS[/caption]
 Dr Natalie Soulsby FPS, Adv Prac Pharm[/caption]
Dr Natalie Soulsby FPS, Adv Prac Pharm[/caption]
 Joanne Gross MPS[/caption]
Joanne Gross MPS[/caption]