Read the full clinical update here.
Get ready to help to protect vulnerable Australians over the age of 60 years from influenza and its most deadly complications.
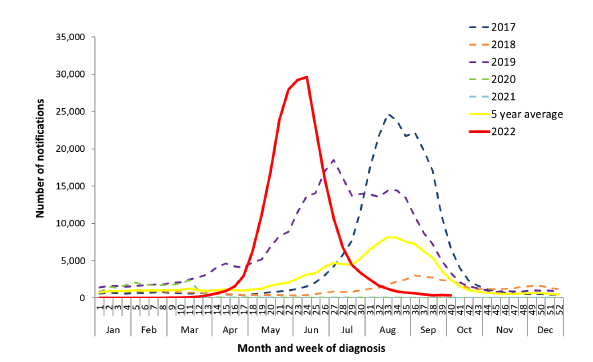
Make no mistake, since Australia re-opened its borders, influenza has made a comeback and poses a risk to our sizeable population over the age of 60 years.1,2 This year has been one of our biggest in the last 6 years in terms of the number of laboratory-confirmed notifications (Figure 1).3 Over 1,800 people were hospitalised with influenza and 300 people lost their lives to influenza.3 Many were over the age of 60.3
Figure 1. Notifications of laboratory-confirmed Influenza in Australia
from 01 Jan 2017 to 09 Oct 20223
This is not unique to Australia. Globally, people over the age of 60 tend to account for many influenza-related hospitalisations and deaths.4 So, what makes people over 60 so vulnerable to influenza complications and death? Both the virus and the body play a role.
The challenge with influenza in older adults is not just the respiratory complications of disease. Cardiovascular events are the most common extra-pulmonary complications of infection with influenza. This includes myocardial infarction, congestive heart failure and strokes, with long-term consequences including cardiovascular disease and cognitive decline.4
In Australia, hospitalisation rates for cardiovascular complications increase during influenza season, with one study showing 12.4% of people admitted with an acute myocardial infarction had unrecognised influenza infection versus 6.7% of controls.5 A study in Canada demonstrated older adults had approximately 5-10x increased risk of myocardial infarction in the first 7 days following influenza infection.6
In addition to acute events, an estimated 15% of older adults admitted with laboratory confirmed influenza will experience disability, with a loss of independence in more than two basic self-care activities.4
How is the influenza virus named?
Influenza viruses can be divided into four subclasses – A, B, C and D. Of these, A and B subclasses are both clinically important as they express two glycoproteins, haemagglutinin (HA) and neuraminidase (NA). Of these, there are 18 known subtypes of HA and 11 subtypes of NA which are important for how different strains are named. Influenza A strains are named after the HA and NA subtypes, while influenza B has two lineages in circulation – Victoria and Yamagata.7 Of all the strains, influenza A (H3N2) has historically been the most problematic for older individuals.8
What makes influenza so dangerous for the over 60s?
People over the age of 60 have a number of additional risk factors for morbidity and mortality related to influenza, including having multiple co-morbidities and an aging immune system (or immunoscenescence).7 In addition, the theory of ‘original antigenic sin’ may play a role. This theory proposes that the first influenza viral antigens a person encounters in infancy will dictate their immune response for the rest of their life. Antibodies from the first influenza vaccine ever received are disproportionately upregulated by subsequent vaccines.7 The reason this happens is because viruses that have mutated by antigenic drift (where genetic material is exchanged between two influenza viruses in the same host cell) retain certain molecular structures, allowing the immune system to use historical plasma cells instead of producing new ones in response to subsequent vaccinations. However, this narrowing of the immune response to vaccinations leaves people at risk if the influenza virus contains larger mutations in these molecular structures.7
The high rate of mutations and genetic reassortment are key to the virus’ ability to cause seasonal epidemics and occasional pandemics. Antigenic drift and shift (where the genetic mutations during replication produce changes in the antigens) are key reasons the virus can cause annual infections and why new vaccines against the latest strains is required.7
What makes vaccination a challenge for older Australians?
Vaccine uptake is still a challenge when it comes to influenza vaccination in Australia. According to the most recent Australian Immunisation Register (AIR; 1 March to 9 October 2022) influenza vaccines have been given to 43% of the population – approximately 11.2 million people.9,10 Of these, 2.7 million were provided in a pharmacy9 – a huge increase from the 10,889 influenza vaccines given as part of the Queensland Pharmacist Immunisation Pilot Program in 2014.11
When it comes to older adults, are all influenza vaccines equal?
It has been recognised that standard-dose unadjuvanted influenza vaccines have reduced efficacy against influenza A (H3N2) in older populations compared to young adults.12,13 Whilst vaccination is an important strategy to help protect the community against complications of influenza infection and can be highly efficacious in young adults (general vaccine effectiveness of 70-90%), vaccine effectiveness in the elderly can be relatively poor (17-53%) and provide shorter duration of protection.7,14 One study demonstrated no increased protection against influenza versus non-vaccination after only 120 days.7,15 This is in part due to the progressive effect ageing has on the innate and adaptive immune responses called immunosenescence.7 This includes downregulation of processes essential for vaccine processing in the body, including cells involved in immune surveillance and activation (Figure 2).16
Figure 2. Mechanisms of immune deficits in older adults16
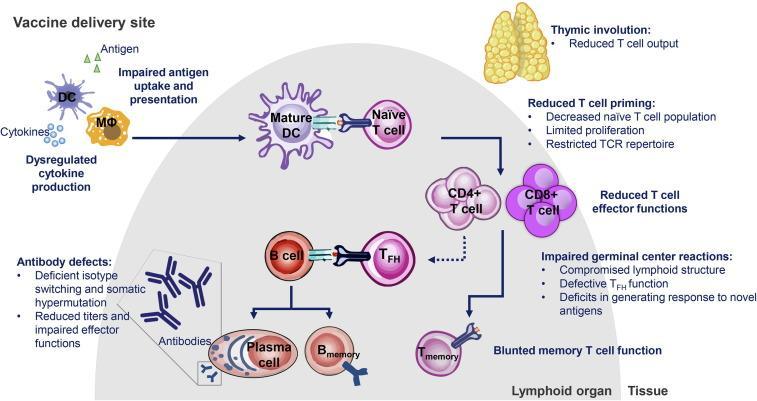
DC=dendritic cell; MΦ=macrophage; TFH=T follicular helper; TCR=T cell receptor.
Immunosenescence is also understood to be linked to comorbidities that are associated with increased influenza disease burden, such as diabetes mellitus, cancer, and other autoimmune and neurological disorders.17
These diminished responses place older individuals at risk of subsequent disease and play a role in the perceived failure of the adaptive immune system to mount an adequate and durable response to vaccination with standard-dose influenza vaccination.18 Influenza vaccines have been designed to stimulate antibody responses to the epitopes surrounding the globular head of haemagglutinin (HA) that permits infection of the host cell.4 While the major protective mechanism of vaccines in young adults is antibody mediated, older adults also need cytotoxic T lymphocyte mediated clearance of the virus once infection occurs.18
To combat the reduced reactivity of the older adult’s immune system, higher immunogenicity vaccines have been developed.12
Fluzone High-Dose Quadrivalent contains 4x the antigen of standard-dose* influenza vaccines and is now available for adults 60 years and over19
*Standard-dose unadjuvanted trivalent influenza vaccine. Standard dose influenza vaccines contain 15 μg of influenza virus HA per strain compared to 60 μg HA of each of the four strains in Fluzone High-Dose Quadrivalent.19
Fluzone High-Dose Quadrivalent contains 4-strain protection, including influenza A(H3N2), A(H1N1), B Victoria lineage and B Yamagata lineage. The indication of Fluzone High-Dose Quadrivalent is based on the demonstration of non-inferior immunogenicity between the quadrivalent and trivalent high-dose vaccines.19
Fluzone High-Dose Quadrivalent offers patients:
More effective in preventing influenza than standard dose* vaccines in adults aged ≥65 years19
*Standard-dose unadjuvanted trivalent influenza vaccine. Standard dose influenza vaccines contain 15 μg of influenza virus HA per strain compared to 60 μg HA of each of the four strains in Fluzone High-Dose Quadrivalent.19
 †Relative vaccine efficacy. The efficacy and effectiveness of Fluzone High-Dose Quadrivalent vaccine in patients aged ≥60 years can be inferred from that for high dose trivalent vaccine in patients ≥65 years, given the demonstration of statistically comparable immunogenicity between both vaccines in both age groups.19
†Relative vaccine efficacy. The efficacy and effectiveness of Fluzone High-Dose Quadrivalent vaccine in patients aged ≥60 years can be inferred from that for high dose trivalent vaccine in patients ≥65 years, given the demonstration of statistically comparable immunogenicity between both vaccines in both age groups.19
More effective in preventing influenza complications than standard dose* vaccine19,20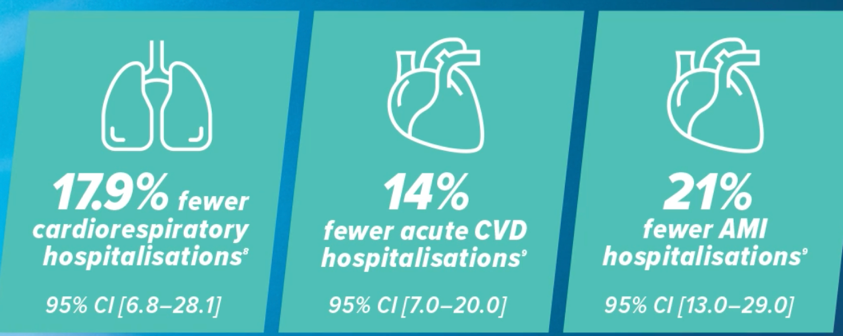
Lee et al. 2018 is a systematic review and meta-analysis of 7 observational studies.20 Van Aalst et al. 2021 is a retrospective cohort study with approximately 700,000 patients.21 AMI: acute myocardial infarction. CVD: cardiovascular disease.
Comparable safety profile with high-dose trivalent influenza vaccine19
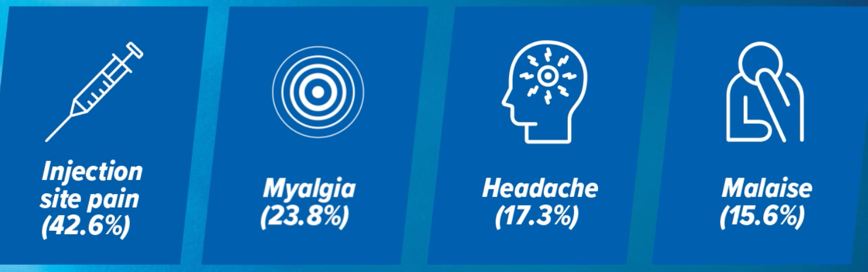
Most common reactions after Fluzone High-Dose Quadrivalent administration. The safety was assessed in a pooled analysis of two clinical trials in 2,549 adults aged 60+.19
When a patient 60 years and over presents for their annual influenza vaccination, there is a high-dose option available that, with 60 μg of each strain, offers more protection against influenza and its complications than standard dose unadjuvanted influenza vaccine with only 15 μg of each strain.19-21
Tips to help you provide high-level protection with Fluzone High-Dose Quadrivalent
- Analyze your 2022 usage to forecast your 2023 season needs
- Place your pre-orders early so you don’t miss out on first deliveries
- Plan your vaccine clinic promotion to make your older community aware you’ll be offering influenza vaccination that’s optimised for them
- Upskill pharmacy vaccination clinic staff on vaccination choice and answering questions on influenza vaccine choices
- Discuss the different influenza vaccine options available with your patients, highlighting the differences between them.
Consider the clinical scenarios
Mary^ has recently turned 60 and has presented for her annual influenza vaccination. Given her proactivity in vaccination and towards her health in general, discussing the high-dose vaccine with Mary is a way to add value to the health care you provide her.
John^ is 65, had a myocardial infarction two years ago and is currently on irbesartan and rosuvastatin. He presents for his repeat prescriptions which is an ideal situation for a quick chat with John about his vaccination intention. He will need to be informed that he is eligible for a NIP funded vaccine and have all of his vaccine options explained to him.
Amanda^ is 70 and taking dapagliflozin for her type 2 diabetes mellitus. She presents for her flu shot in your pharmacy. Given Amanda’s age and comorbidity, there is a chance a standard dose vaccine could have limited effectiveness. Amanda is eligible for a NIP-funded vaccine, but consider discussing all the higher-immunogenicity influenza vaccine options that offer additional protection from influenza compared to standard-dose vaccines.
Charles^ is 63 and is looking at over-the-counter cold and flu medications. This provides an opportunity to proactively discuss the benefits of influenza vaccination as the simplest and most effective way for Charles to protect himself against influenza. The various options can be discussed, including a high-dose influenza vaccine specifically for Charles’ age group that can offer additional protection.
^Hypothetical patients. Fluzone High-Dose Quadrivalent is not on the NIP. Refer to the NIP schedule for funded influenza vaccines.
References:
- Australian Institute of Health and Welfare. Older Australians. Web report, last updated 30 Nov 2021. Available from: https://www.aihw.gov.au/reports/older-people/older-australians/contents/summary (accessed November 2022).
- Immunisation Coalition. Influenza Statistics. Page published 7 February 2021. Available from: https://www.immunisationcoalition.org.au/news-data/influenza-statistics/ (accessed November 2022).
- Australian Government Department of Health and Aged Care. Australian Influenza Surveillance Report No. 14, 2022. Reporting fortnight: 26 September to 09 October 2022.
- McElhaney JE, et al. Immun Ageing 2020:17:10.
- MacIntyre CR, et al. Heart 2013;99(24):1843-1848.
- Kwong JC, et al. N Engl J Med 2018;378(4):345-353.
- Tanner AR, et al. Eur Respir Rev 2021;30:200258.
- Jester BJ, et al, Am J Public Health 2020;110(5):669-676.
- Australian Government, Australian Immunisation Register (AIR) Influenza (flu) immunisation data – 1 March 2022 to 9 October 2022.
- Australian Bureau of Statistics, National state and territory population. Reference period June 2022, released 15 December 2022. Available from: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release (accessed December 2022).
- The Pharmacy Guild of Australia, Vaccination Services. History of Pharmacy Vaccination Services. [Internet]. Available from: https://www.guild.org.au/programs/vaccination-services (accessed November 2022).
- Monto AS, et al. Vaccine 2009;27(37):5043-5053.
- Public Health England. Influenza: The green book, Chapter 19. Available at: https://www.gov.uk/government/publications/influenza-the-green-book-chapter-19 (accessed August 2021).
- Goodwin K, et al. Vaccine 2006;24:1159-1169.
- Castilla J, et al. Euro Surveill 2013;18:20388.
- Allen JC et al. Vaccine 2020;38(52):8264-8272.
- Zagaria MAE, US Pharm 2020;45(10:10-12.
- Smetana J, et al. Hum Vaccin Immunother 2018;14(3):540-549.
- Fluzone Product Information.
- Lee JKH, et al. Vaccine 2021;39 Suppl 1; A24-A35.
- van Aalst R, et al. Vaccine 2021;39 Suppl 1;A51-A55.
| PBS Information: This product is not on the PBS or National Immunisation Program. |
Please review full Fluzone High-Dose Quadrivalent Product Information before prescribing. Full Product Information is available on request from Sanofi on 1800 818 806 or online here.
sanofi-aventis Australia pty ltd. ABN 31 008 558 807. Trading as SANOFI PASTEUR. 12-24 Talavera Rd, Macquarie Park NSW 2113. Customer Service Ph: 1800 829 468. MAT-AU-2203156. SAIN27807W. Date of preparation: January 2023.



 John Jones MPS, pharmacist immuniser and owner of My Community Pharmacy Shortland in Newcastle, NSW[/caption]
John Jones MPS, pharmacist immuniser and owner of My Community Pharmacy Shortland in Newcastle, NSW[/caption]


 Debbie Rigby FPS explaining how to correctly use different inhaler devices[/caption]
Debbie Rigby FPS explaining how to correctly use different inhaler devices[/caption]




 Professor Sepehr Shakib[/caption]
Professor Sepehr Shakib[/caption]

 Lee McLennan MPS[/caption]
Lee McLennan MPS[/caption]
 Dr Natalie Soulsby FPS, Adv Prac Pharm[/caption]
Dr Natalie Soulsby FPS, Adv Prac Pharm[/caption]
 Joanne Gross MPS[/caption]
Joanne Gross MPS[/caption]








