The Pharmaceutical Benefits Scheme (PBS) recently made changes to the listings for proton pump inhibitors (PPIs) in an effort to address overprescribing.
The changes, which came into effect on 1 May 2019, affect prescribing of esomeprazole, lansoprazole, omeprazole, pantoprazole and rabeprazole, for the treatment of gastro-oesophageal reflux disease, peptic ulcers, and hypersecretory conditions such as Zollinger-Ellison Syndrome and scleroderma oesophagus.1
At a March 2018 meeting, the Pharmaceutical Benefits Advisory Committee, which found that ‘in comparison to the clinical guidelines and considering the prevalence of gastro-oesophageal reflux disorders, high dose PPIs appear to be overprescribed in Australia, for excessively long periods of time, particularly amongst older people.’2
Changes to terminology, authorisation and clinical criteria
The terminology for PPI doses has changed from ‘highest’, ‘high’, and ‘low’ to ‘high’, ‘standard’, and ‘low’. Because of its higher therapeutic relativity, prescriptions for 40 mg esomeprazole (with one repeat) are now Authority Required (Telephone).
Similarly, all standard dose PPIs are now Authority Required (Streamlined).1
The clinical criteria for the 40mg dose of esomeprazole has also changed, such that patients must have symptoms that have not been adequately controlled by a standard dose of PPI. The PBS listing also advises checking patient adherence to the lower dose PPI before stepping up to the higher dose.
At the same time, the GORD indication has been removed from item numbers for standard dose PPIs used to treat other gastrointestinal acid-related disorders.1
Stepping down
Stopping PPIs abruptly can lead to rebound acid hypersecretion. Tapering approaches seem to be the best option – an NPS MedicineWise algorithm recommends stepping down from standard to low-dose after 4–8 weeks. Other discontinuation strategies include the use of H₂-receptor antagonists, intermittent on demand PPI, or antacids. For more information about PPI discontinuation, refer to the recent June edition Australian Pharmacist CPD article: Proton Pump Inhibitor deprescribing.
Advice for patients
Acid reflux and heartburn are common symptoms. They can be mild and intermittent, occurring no more than once a week, or severe and persistent, leading to a diagnosis of gastro-oesophageal reflux disease (GORD), where symptoms can significantly impact a patient’s quality of life.
Reflux symptoms can be triggered by spicy, rich or fatty foods, or by eating too much or too quickly. Caffeine and alcohol can also precipitate symptoms. Pharmacists have a role to play by advising individuals to make dietary and lifestyle changes to manage their symptoms, even when taking a PPI. Lifestyle factors include losing weight, stopping smoking, cutting back on alcohol, raising the head of the bed, avoiding lying down after eating, eating smaller meals, avoiding food before exercise and avoiding eating 2–3 hours before bedtime.
Patients taking PPI therapy for GORD should be made aware that their therapy will need periodic reviewing after an initial course of treatment. Long-term unnecessary use of PPIs should be avoided if possible.This is an opportunity to introduce the concept of stepping down. Stepping down could involve lowering the dose, taking the PPI only when they get symptoms, or stopping the PPI.
References
- NPS MedicineWIse. Proton pump inhibitors: PBS changes May 2019. At: https://www.nps.org.au/radar/articles/proton-pump-inhibitors-pbs-changes-may-2019?utm_medium=email-nps&utm_source=npsc_41ec83d55f&utm_campaign=mwupdate&utm_content=other-hp
- Pharmaceutical Benefits Scheme (PBS) 2018 PBAC Outcomes. At: http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2018-03/other-matters-03-2018.pdf
- Pharmaceutical Benefits Scheme (PBS) Fact Sheet: Streamlined Authorities. At: http://www.pbs.gov.au/info/publication/factsheets/shared/fact-sheet-streamlined-authorities



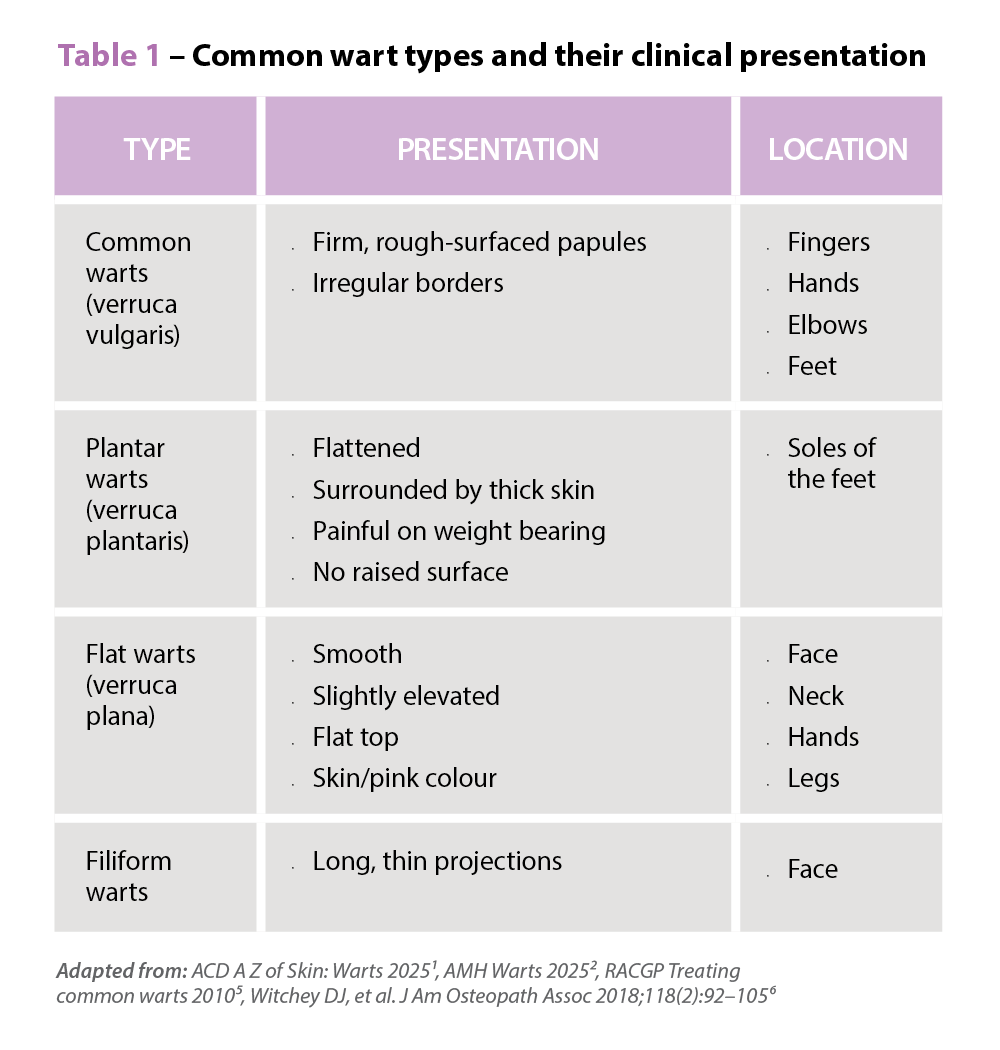 Symptoms
Symptoms