As the detail of the Seventh Community Pharmacy Agreement is worked out, five subject matter experts share their thoughts so far.
Pharmacy in Australia is expected to undergo evolutionary change during the next 5 years of the Seventh Community Pharmacy Agreement (7CPA) and its $1.2 billion worth of patient-focused pharmacy programs.
After a horror start to the year with unprecedented bushfire damage, loss of life and pharmacists stepping up to the plate to provide essential help to their communities, the COVID-19 pandemic has spurred new regulations, legislation and other advances for the profession at a pace that continues to take many by surprise as the fluid situation of community transmission of the virus continues.
As a consequence of this rapid change, the sending of Australia’s first legally valid electronic prescription – in Victoria in May and followed elsewhere including in Tasmania in July – heralds the shift away from manually intensive paper prescriptions and less work around dispensing for Australian pharmacists.
Aboriginal and Torres Strait Islanders will benefit from several changes to relevant programs.
Quality Use of Medicines
The 7CPA will bolster the quality use of medicines (QUM) with the aim of improving the health outcomes of all Australians, in line with PSA’s Pharmacists in 2023 report of last year. The uptake of electronic prescriptions is part of the pipeline of initiatives endorsed by PSA, a signatory to the agreement for the first time.
Australian Pharmacist asked subject matter experts to give their views on the broad details of the 7CPA known so far in relation to specific program areas, including those related to rural regions, and the health of Aboriginal and Torres Strait Islander people and the ceasing of the clinical interventions program.
And the funding of up to two follow-up visits after a Residential Medication Management Review or Home Medicines Review continues to make significant differences in health outcomes.
Here’s what the experts think.
7CPA key points
|
Clinical interventions program
Greg Peterson FPS
Distinguished Professor of Pharmacy, University of Tasmania, and community pharmacist
It was not surprising that the clinical interventions program was removed as part of the 7CPA, according to Distinguished Professor Greg Peterson, who reviews NHMRC Project and Development Grant applications and was appointed by the Australian Research Council as an expert panel member for health and medical research in judging Excellence in Research in Australia.
‘I fully agree with the decision. It was either a matter of deleting the program or re-structuring it to meet the original intent,’ he said.
The initial development of the clinical interventions program, which was part of almost 10 years of research prior to its implementation, was based on the model of compiling a central repository of interventions performed within Australian community pharmacy.
In the trials preceding the implementation of the funded program in the 5CPA, which began in 2010, pharmacists’ interventions were sent electronically to a secure repository. Such a large dataset had a variety of potential uses.
‘In the trials, it was used to indicate to individual pharmacies their rates of interventions compared to state and national averages.
‘Electronic prompts linked to dispensing were also developed, for instance to promote discussion around reducing dosages/deprescribing of proton pump inhibitors,’ Professor Peterson recalled.
The data could also be used for pharmacovigilance purposes, such as reports of adverse reactions to new medicines. Another use was for CPD purposes – as a log of pharmacists’ personal clinical activity and as a source of educational cases and vignettes.
Perhaps most importantly, Professor Peterson pointed out, the data could be used, as in the trials, to provide a large bank of pharmacists’ interventions that could be randomly sampled and subjected to expert clinical panel and economic analysis.
‘This would have largely addressed what still remains as probably our profession’s single major Achilles’ heel – the lack of robust evidence to convince policymakers and other health professionals that we actually make a difference in clinical and health economic terms.’
But the recommended model of the clinical intervention program was not rolled out.
According to Professor Peterson, the profession has suffered as a result.
‘It is my hope that the professional services in the 7CPA will have measurable and robust outcomes attached, and that the data was pooled centrally to enable rigorous assessment and to guide the development of further services.’
Simply ticking boxes, he said, and not compiling and analysing nationally collected data, is pointless. Nor would it advance either the profession or medicine safety for the Australian public.
Rural programs
Peter Crothers FPS
is the only pharmacy owner in Bourke, NSW
Last year’s PSA Pharmacist of the Year likes to think of himself as an optimist.
So Mr Crothers is keen to learn some detail – any detail – about just what difference the change in models of ‘remoteness’ under the 7CPA will mean for very remote pharmacies, like his.
The movement from the Pharmacy Accessibility/Remoteness Index (PhARIA) to the Modified Monash Model (MMM) will likely classify as inaccessible previously classified ‘accessible’ cities such as Bendigo, Dubbo, Tamworth, Toowoomba and Bundaberg.
‘What we know is that the Rural Pharmacy Maintenance Allowance money is somewhat greater than it was in the last agreement, but it is to be shared among a greater amount of pharmacies, it appears,’ he says, referring to the mooted 800 extra pharmacies it may cover.
Mr Crothers would also like some detail on rural workforce funding and how that might change in the 7CPA as it is a ‘major issue for all pharmacies that are not classified as accessible’.
Smaller NSW towns including Canowindra, Hillston, Nyngan – even Narromine outside Dubbo – are considered ‘inaccessible’ or remote. And Bourke, formerly a 6 on PhARIA is a 7 in the MMM, in ‘the most remote’ category.
‘We need an absolute radical overhaul of workforce assistance in these areas,’ he says. ‘In terms of dollars spent per hours work gained from a pharmacist employee, it costs me almost triple what it costs to employ a pharmacist in the metropolitan area of the more accessible areas.’
‘Unlike, say, high rents in city pharmacies that are offset by increased store traffic and sales, these higher rural labour costs are not offset – by anything, Mr Crothers says.
‘They’re just a dead weight on the business and that’s a major issue.’
Quality Use of Medicines
As the agreement rolls out, it is intended to be structured around the quality and safe use of medicines.
But, Mr Crothers points out, amid ‘a lot of talk about deprescribing’ – of opioids, proton pump inhibitors, sedatives – there were ‘no rewards for deprescribing’ in the 7CPA.
Professional programs
Although the 7CPA provides more money for professional programs than its predecessor Mr Crothers believes ‘nobody’s got a blind idea how that’s going to work out’.
‘We are told,’ he said, ‘that some of the caps are going to be removed on Aboriginal programs, for example dose administration aids (DAAs) and that’s a very good thing, and that they’re going to be properly funded – funded at an amount that approximates the actual cost, and that’s a really good thing.’
It remains to be seen what unfolds in the next 6 months, Mr Crothers says, as the failure to recognise rural workforce difficulties is one of the structural issues that needs urgent attention.
Without some kind of quality framework or funding model to reward activities like deprescribing, it would be hard to achieve a fundamental improvement in medicine safety, Mr Crothers warns.
Aboriginal and Torres Strait Islander Programs
Mike Stephens MPS
Director, Medicines Policy and Programs, National Aboriginal Community Controlled Health Organisation.
Hannah Loller MPS
Manager of the PSA’s Integrating Pharmacists into Aboriginal Community Controlled Health Services Project (IPAC), which recently completed its trial and reported to the federal government on 30 June.
Aboriginal and Torres Strait Islander health has received a boost in the 7CPA with increased focus on programs and evolutionary changes foreshadowed to support Australia’s first people.
Mike Stephens is looking forward to further detail on 7CPA programs relating to this important health area.
‘Some challenges persist for Aboriginal and Torres Strait Islander people in accessing medicines and that government spending on Pharmaceutical Benefits Scheme (PBS) medicines is well below that of other Australians – so more needs to be done,’ he said.
‘Self-determination through community control is a critical component of any measures that aim to improve medicines access and use for Aboriginal and Torres Strait Islander peoples.’
As such, he said, relevant 7CPA programs must respect Aboriginal Community Controlled Health Organisation (ACCHO) models of care and their respective priorities.
A ‘blueprint for reform’ already exists in this area through the Review of Indigenous Pharmacy Programs (IPPs), Mr Stephens said, in addition to the Senate Inquiry into the s100 Remote Area Aboriginal Health Services (RAAHS).
Ms Loller, while welcoming the addition of uncapped dose administration aids (DAAs), pointed out that the devil is always in the detail.
Also welcome are modifications in relation to the Closing the Gap (CTG) Pharmaceutical Benefits Scheme (PBS) co-payment measure, which will enable more health professionals the ability to register patients for the measure – regardless of their geographic location.
Current programs that aim to improve access to PBS medicines, i.e. s100 RAAHS supply and CTG are different, depending on the geographical location of individual patients, Ms Loller said.
‘This is confusing and leads to challenges as patients move between physical locations. It has been identified as an issue for a long time.
‘Modifications need to ensure access to medicines is facilitated at the most appropriate point of care, in a culturally safe environment.
‘This might be at the health service rather than a community pharmacy, e.g. remote locations where no community pharmacy exists or a patient attending an urban/regional-based Aboriginal health service for the management of rheumatic heart disease to have benzathine benzylpenicillin administered.’
Nor, Ms Loller added, is it clear ‘what impact the proposed CTG changes intend to have regarding access to medicines at the point of discharge from a hospital, a critical transition of care juncture, with known challenges for Aboriginal and Torres Strait Islander patients.’
It will be important to ensure proposed program modifications respect and reflect the recommendations arising from a number of reviews over the last decade, relating to IPPs, Ms Loller said.
‘Hopefully, program changes will see a more equitable distribution of the funding intended to support much needed QUM for both individuals and services, as recommended in the IPP review. The review of Community Pharmacy Programs will provide an opportunity to advocate for relevant program rule changes, e.g. enable HMRs to be undertaken without the need to seek prior approval for a review to be conducted outside a patient’s home,’ said Ms Loller.
‘This program rule is a well-known and documented barrier for Aboriginal and Torres Strait Islander patients accessing a comprehensive medication review service.’
RMMR follow-up visits
The 7CPA has incorporated up to two remunerated follow-up visits to a Residential Medication Management or Home Medicines Review – following recommends in October last year in the interim report of the Royal Commission into Aged Care Quality and Safety. Here is one example of how follow-up visits to an RMMR have already improved patient care.
Andrew Stafford MPS
Consultant pharmacist and senior lecturer in pharmacy practice, Curtin University, Western Australia.
Problem at initial review
Gloria*, a new resident in a residential aged care facility, had advanced dementia. Despite the nursing staff’s best efforts to orientate her and address her needs, Gloria had been highly aggressive towards other residents, for which she commenced quetiapine shortly after admission. After 3 months on 100 mg of quetiapine nightly, Gloria had not been aggressive towards other residents or staff for several weeks. But she was increasingly drowsy in the mornings and had developed a tremor, which made eating with utensils difficult.
Advice
An RMMR for Gloria identified that withdrawal of the antipsychotic should be considered in view of her stable behaviour, and the potential for it to cause adverse effects. Care staff, however, were reluctant to withdraw the quetiapine based on her previous aggression.
Recommendation
A discussion between care staff, the GP and a representative of Gloria’s family resulted in agreement that the quetiapine would continue for another month, with ongoing monitoring for benefit and adverse effects. A follow-up RMMR visit was also scheduled to reassess Gloria’s use of quetiapine throughout this time.
Follow-up 1
The first follow-up service was undertaken 5 weeks after Gloria’s RMMR. Since then, she had experienced no episodes of severe aggression and was asleep most mornings. Gloria’s family representative and the care team agreed at this visit to begin a downward titration of the quetiapine, with a view to eventual cessation. A second follow-up service was scheduled for 2 months’ time to evaluate the effect of the quetiapine dose reduction/withdrawal.
Follow-up 2
Two weeks prior to the second follow-up service, Gloria had ceased taking quetiapine entirely. While more alert and occasionally inappropriately vocal, she had not been aggressive towards other residents or staff. No member of Gloria’s care team believed she would benefit from the reintroduction of an antipsychotic, and her family representative was pleased Gloria was more engaged. Her tremor remained, albeit greatly lessened, and she was able to use eating utensils again.
Comment
Without the follow-up services, it is possible Gloria may have remained on quetiapine for many more months. Despite the intention for the quetiapine to be reassessed by the care team some weeks after the initial RMMR, this can often be overlooked. Had that occurred, it would have prolonged Gloria’s sedation and tremor, and adversely affected her quality of life. She was also at increased risk of serious adverse effects from the extended duration of quetiapine treatment. Instead, the follow-up services provided valuable opportunities to formally reassess Gloria’s situation much more frequently than would have been possible under the former RMMR payment arrangements.
Reference
- Australian Government Department of Health. PBS expenditure and prescriptions report 1 July 2018 to 30 June 2019. At: pbs.gov.au/info/statistics/expenditure-prescriptions/pbs-expenditure-and-prescriptions-report
*not her real name
| To read more about 7CPA, including information on dispensing remuneration from Bruce Annabel, Consultant to Pitcher Pharmacy Services, Adjunct Professor of Pharmacy Management at Queensland University of Technology, see the August issue of the Australian Pharmacist journal. |




 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.

 Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4
Beyond the arrhythmia, AF often signals broader pathological processes that impair cardiac function and reduce quality of life and life expectancy.5 Many of these conditions are closely linked to social determinants of health, disproportionately affecting populations with socioeconomic disadvantage. Effective AF management requires addressing both the arrhythmia and its underlying contributors.4  C – Comorbidity and risk factor management
C – Comorbidity and risk factor management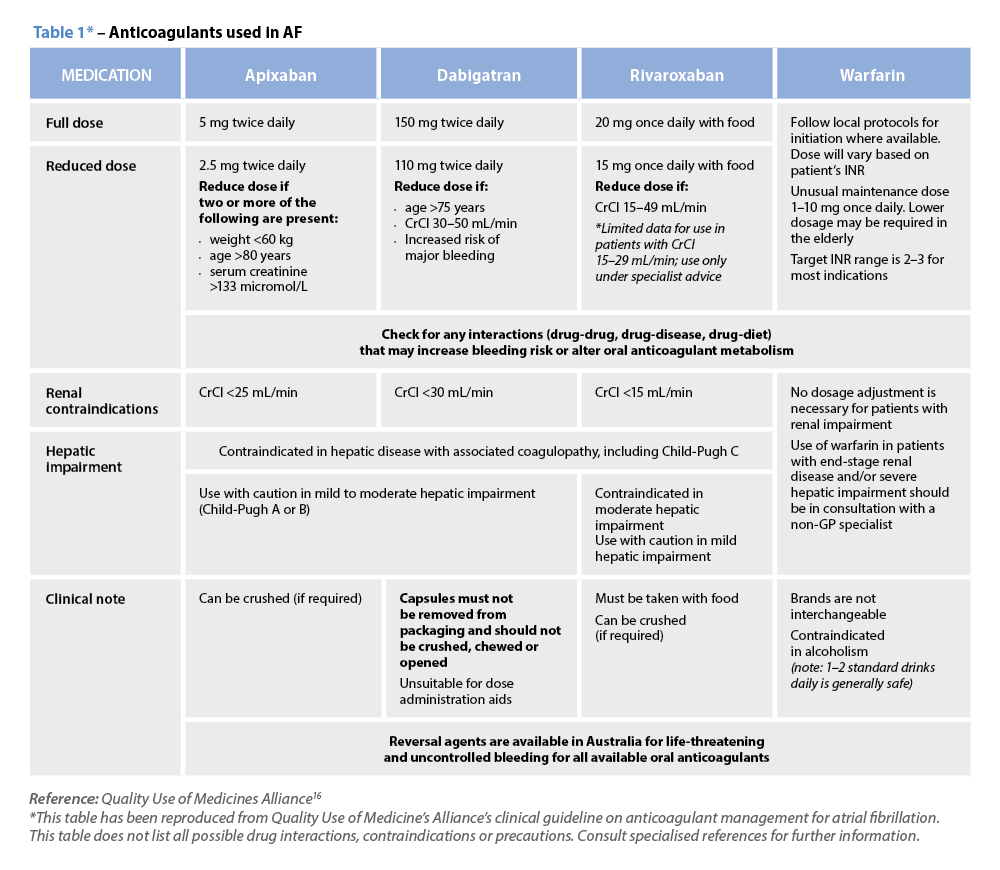 Warfarin
Warfarin




