Pharmacists should be able to immunise for more diseases, as well as for COVID-19.
On the morning of 7 September 2020, the Prime Minister Scott Morrison announced that two COVID-19 vaccines would be made available to all Australians next year free of charge, if trials were successful and proven safe and effective.1
That same day, PSA wrote to all state and territory governments, urging them to amend legislation to ensure pharmacists would be permitted to administer any approved COVID-19 vaccine.
Two months later, Australia signed up for another two potentials – including the promising Pfizer mRNA vaccine – that will boost federal government funding of immunisation to $3.2 billion, if the vaccines prove efficacious and are approved for use. Federal Health Minister Greg Hunt says Australians who want a vaccination should get it ‘within 2021’.2
A new national COVID-19 Vaccination Policy sets out the responsibility of government at all levels, priority groups to be vaccinated and vaccination sites.3
Pharmacists are ‘likely to play a role in COVID-19 vaccination for some part of the population (e.g. healthy adults) at some stage, depending on the safety profile of the vaccines,’ according to the policy.
But what about pharmacists administering vaccines for other diseases?
Artificial barriers
The PSA’s Queensland-based General Manager Policy and Program Delivery Chris Campbell says artificial barriers are still preventing the rollout of more pharmacy-delivered vaccines for a wide range of preventable diseases in Australia.
‘It is absurd,’ Mr Campbell tells AP.
‘Pharmacists can competently administer intramuscular and subcutaneous injections, as demonstrated by the training they have done, but why for some vaccines and not for others?’
The barriers are political and bureaucratic in nature and bolstered by special interest groups within healthcare professions, he says.
Mr Campbell adds: ‘If herd immunity for a range of diseases, including COVID-19, is to be achieved, then it’s pharmacists who are ideally placed to deliver vaccination programs on a major national scale.’
Complete, accurate and timely data will be necessary to determine the uptake and effectiveness of a COVID-19 vaccine, as well as mandatory recording under the new vaccination policy.
And ‘as a workforce at increased risk of exposure, pharmacists (and pharmacy staff) are expected to be part of the priority group’ of initial coronavirus immunisations’, Mr Campbell says.
2020COUNTDOWN TO A COVID-19 VACCINE |
| FEBRUARY 2020
CSL begins testing a vaccine developed in just 3 weeks by University of Queensland (UQ) researchers. |
| MARCH 2020
Numerous pre-clinical studies underway for protein-based, viral vector, mRNA and nucleic acid vaccines to prevent COVID-19. |
| MAY 2020
WHO announces launch of coordinated, international, concurrent randomised controlled Phase 3 trial – SOLIDARITY – of different vaccine candidates aimed at enrolling 280,000+ participants from at least 470 different sites in 34 countries with interim results due in January 2021. |
| SEPTEMBER 2020
Australia signs agreement for 50 million doses of an Oxford University/AstraZeneca vaccine and 33.8 million doses of the UQ/CSL vaccine. |
| OCTOBER 2020
42 COVID-19 candidate vaccines in clinical evaluation, 10 in Phase 3I trials and most designed for two doses. |
| NOVEMBER 2020
Federal government signs up for 40 million doses of Novovax protein vaccine and 10 million doses of the Pfizer/BioNTech mRNA vaccine, subject to TGA approval for efficacy and safety. CSL began manufacturing millions of vials of one of the University of Oxford/AstraZeneca vaccines, ahead of trials that may demonstrate its efficacy and TGA approval, for a potential rollout from March 2021. Federal government COVID-19 Vaccine Policy launched. |
Legislative differences
The number of vaccines pharmacies are permitted to deliver in each state and territory, and to what age group, varies in fractured and complex legislative frameworks.
In Queensland, for instance, pharmacists can administer 11 types of vaccine for patients 16 years and over, including the flu and meningococcal vaccines for anyone over the age of 10.
The vagaries of legislation in different states and territories show the benefit of unity on the issue of pharmacist-administered vaccination legislation, as well as an expanded list of preventable disease immunisations that can be administered by pharmacists. The 7CPA also pledged to work towards consistency in pharmacy-administered vaccines across the country.
PSA National President Associate Professor Chris Freeman says with PSA’s proactive approach to legislative amendments when a COVID-19 vaccine does become available, all jurisdictions around Australia should be ready to go.
Queensland has already acted. Before its recent re-election, the Palaszczuk Government amended the required drug therapy protocol for pharmacists there to administer a COVID-19 vaccine, when one becomes available.
‘As the first state to provide pharmacist-administered immunisations since 2014, it would be safe to say that the majority of pharmacies in Queensland are equipped with a clinic room and offer immunisation,’ says PSA’s Chris Campbell.
‘We are also seeing hospitals offering services in outpatient clinics and more pharmacists adding consult rooms during shopfits of their businesses to provide this service.’ Queensland regulation, he points out, also allows for students to immunise under direct supervision, ‘meaning all four pharmacy schools in the state provide immunisation qualifications by the time students graduate’.
The upshot is ‘a growing of the immunisation workforce’, he says, with dual outputs from graduates who are immunisation-ready and registered pharmacists continuing to undergo immunisation training.
In May this year, the National Centre for Immunisation Research and Surveillance (NCIRS) released its report on the Review of pharmacist vaccination reporting to the Australian Immunisation Register.4
The research that informed the report found that although pharmacist-administered vaccinations have grown considerably in recent years, they still accounted for less than 3% of all vaccinations given in Australia in 2019.
‘We estimate that’s a significant under-reporting,’ NCIRS Associate Director Dr Frank Beard says. ‘The proportion was probably higher than that, and we expect that proportion to have increased in 2020.’
Dr Beard says the ability to track those who had been vaccinated, their location and the outcome – through linkage of AIR to other data sources – would provide information that is critical to monitoring how the vaccination program progresses.
The NCIRS report concluded that the lessons learned, and the systems implemented during the pandemic, could be leveraged to improve routine pharmacist vaccination services and strengthen the position of pharmacists as immunisers.
Pharmacists only gained access to software fully integrated with the Australian Immunisation Register (AIR) in 2019.
Pharmacists who use software integrated with the AIR can access training and support from their provider to ensure they are sending the encounters correctly and they reach their destination, according to the NCIRS.
Encounters can also be uploaded directly through the AIR’s online platform.
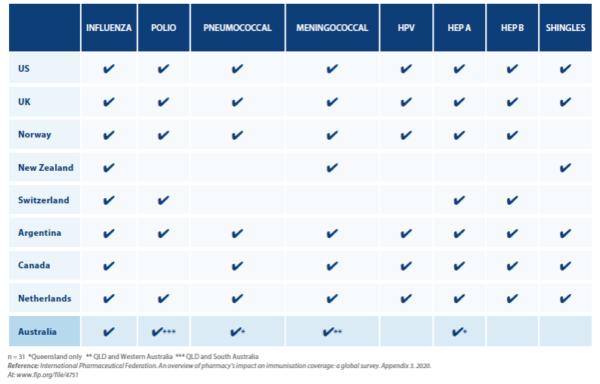
TABLE 1 – Pharmacist vaccination for most commonly immunised conditions in selected countries compared with Australia
NCIRS REPORT RECOMMENDATIONS3:
|
International trend
An International Pharmaceutical Federation (FIP) study released earlier this year found the number of countries and territories that now permit pharmacist-administered vaccinations has almost doubled to 36 in the past 4 years, providing services to almost 1.8 billion people.5 And while that number is expected to double again by 2025, there are many countries that have signalled no intention to introduce pharmacist-administered vaccinations in the foreseeable future. These include Italy, Spain, Japan, China, India and Russia.
The report highlighted the disparity between high- and low-income countries. Pharmacy-based vaccinations were available in almost 50% of high-income countries, but in low-income countries the rate of uptake was just 11%.5
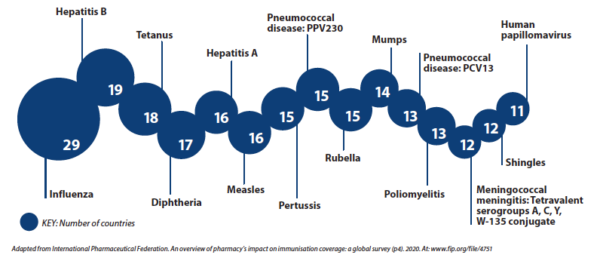
Influenza is the most common vaccine administered by pharmacists worldwide, followed by hepatitis B, tetanus and diphtheria.
Recently the International Pharmaceutical Federation (FIP) called on governments and other stakeholders to take urgent action to ensure equity in access to disease prevention measures, including greater investment in vaccines and vaccination services by community pharmacists.
FIP CEO Catherine Duggan appealed for developments in regulatory frameworks, appropriate remuneration models to support and sustain a broader role for community pharmacies and education and training of the pharmacy workforce to increase vaccination coverage rates.
‘This call is particularly urgent as vaccines against COVID-19 enter advanced stages of clinical research, and mass immunisation strategies will be required not only to protect the most vulnerable population groups, but to avoid additional lockdowns and economic paralysis.’6
The 15 vaccines most commonly administered by pharmacists around the world
Mass rollout
In September, the federal government called for tenders from independent providers to assess the performance of the 2020 flu vaccination season, in an effort to weed out supply chain issues and other problems ahead of what will become the country’s largest mass vaccination program.
Also in September federal Minister for Health Greg Hunt announced that as part of the government’s strategy to secure early access to any safe and effective vaccines, it had joined the COVAX Facility, which enables the purchase of COVID-19 vaccine doses as they become available from a large portfolio of COVID-19 vaccine candidates and manufacturers.7
The COVAX Facility was established by the Vaccine Alliance as part of an international vaccine partnership with the World Health Organization, the Coalition for Epidemic Preparedness Innovations and other bodies. In August, Australia donated $80 million to the COVAX Advance Market Commitment, which will provide doses to developing countries in a collaborative effort to help protect their most vulnerable groups.
Under the September agreement with the COVAX Facility, which opens up additional supplies for Australia from which access may be possible for up to half the population under a two-dose treatment requirement, Australia committed an initial $123.2 million to be part of the purchasing mechanism of the facility. Australia can receive offers to buy vaccines when they become available, while the purchase of doses will be negotiated as they meet the standards of safety and effectiveness of the Therapeutic Goods Administration (TGA).
‘Whoever finds a COVID-19 vaccine must share it. Australia signing up to the COVAX Facility is an important part of our commitment to this principle,’ Mr Hunt says.7
‘Being a part of COVAX means we’re giving Australians the best chance of accessing a safe and effective vaccine, but also our neighbours in the Pacific and Southeast Asia, and partners overseas.’
Even if more vaccines in Phase 3 trials prove effective, herd immunity in Australia will not be achieved without a reliable supply chain. To date, Australia has signed heads of agreement on four potential COVID-19 vaccines.1,2 In the September agreement, it signed up for 50 million doses of the University of Queensland/CSL vaccine now being manufactured ahead of approval, and more than 30 million doses from a similar University of Oxford/AstraZeneca trial. Both would likely require two doses.
Last month, under the newest heads of agreement signed,2 Novavax will supply 40 million doses of its protein-type vaccine for which Phase 3 trials are now underway in the United States and Mexico. As well, should it also prove effective, another 10 million doses of the Pfizer/BioNTech mRNA vaccine, which contains genetic material that enables the growth in participants of the ‘spike protein’ associated with the SARS-COV-2, will become available. Subject to approval by the TGA, both vaccines are expected to be available in early/mid 2021.
‘The goal and the expectation is that Australians who sought vaccination will be vaccinated within 2021,’ Minister Hunt says.
In September, even before its marathon Stage 4 lockdown of Melbourne ended, the Andrews Government had begun identifying mass vaccination venues across its capital, singling out town halls and sporting facilities as viable places where thousands could be immunised daily.
Victorian health advisers and bureaucrats have been brainstorming how to upskill enough health workers to deliver the vaccine.
To enable more pharmacists to be trained, and upskill those already trained in vaccine administration, federal and state governments must liaise with existing providers of training in immunisation to pharmacists, says the NCIRS. And this will be chiefly through PSA and the Pharmacy Guild of Australia.
In the meantime, Mr Campbell cites four key drivers that will help increase immunisation by pharmacists:
- Providing pharmacists access to National Immunisation Program (NIP) stock
- Subsidising the provision of immunisations by pharmacists via the Medicare Benefits Schedule or similar funding
- Allowing pharmacists to administer immunisations for all ages and all conditions subject to vaccination.
He points out that a ‘largely unseen’ result of preventing NIP access to pharmacists is that the adjuvanted influenza vaccine recommended for the vulnerable group of people over 65 is unavailable to them by pharmacists (except in Victoria, Western Australia and the ACT) ‘even in the private market’ and they must ‘jump through hoops to get it through current providers’.
‘Immunisation is a clear public health benefit and pharmacists being able to utilise this skill every day are feeling more and more fulfilled in their role, particularly the contribution they are making as part of the broader health care team,’ says Mr Campbell.
‘What it also encourages is confidence in administering other medicines and a desire to continue to strive for full scope.’
New challenges
In the event of a COVID-19 vaccine rollout in Australia in 2021, there will remain additional and significant challenges for pharmacist immunisers. As vaccination approaches critical mass, preferences may change in favour of booked clinics or additional dedicated resources.
A Netherlands study published in July 2020 found the COVID-19 had had a considerable impact on the logistical procedures and services provided by pharmacists in that country, particularly in relation to patient education and counselling.8
The findings prompted the NCIRS to draft a letter to the Research in Social and Administrative Pharmacy journal, noting that in Australia the COVID-19 pandemic had adversely affected the ability of pharmacists to provide vaccination services.9 Almost all (96%) of the 226 Australian pharmacists who responded to an NCIRS survey conducted in June 2020 say they experienced a higher-than-expected demand for influenza vaccinations in their pharmacies. And while this required adjustment and caused private market vaccine shortages, it may be a signal for the future of mass vaccination programs.
The NCIRS’s Dr Beard, a co-author of the letter, says almost half of those affected needed to implement new protocols such as additional cleaning regimes that meant extra time per patient had to be allotted as these measures took away time to administer services.
Distancing requirements limiting the number of staff and patients that could be on the premises at any given time also added to workloads, as 1 in 10 pharmacists stopped accepting walk-in patients and introduced new booking systems.
Recruitment of qualified staff to safely administer the vaccinations was also a challenge for some operators.
Dr Beard tells AP that while pharmacists could play a key role in administering an eventual COVID-19 vaccine, the new opportunities would also bring additional challenges.
‘Obviously the vaccine will be the main path out of the pandemic, so everyone is waiting with bated breath,’ he says. ‘Hopefully, at least one or possibly more than one vaccine will be available by early next year.’
Dr Beard says Australians need to accept the reality that in the early stages of the vaccine rollout there would be a limited supply and there will need to be priorities set – for people such as healthcare workers exposed regularly to the virus, and vulnerable elderly people.
‘Of course, the plan will be to vaccinate as many people as soon as possible to obtain herd immunity,’ he says. Critical to the rate in which Australia will achieve herd immunity is whether the vaccine will require one or two doses per patient.
‘It’s not simple to calculate what proportion of the population need to be vaccinated; it depends on the nature of the vaccine and how effective it is,’ he says.
‘It could be that the vaccine we end up with doesn’t stop people from contracting COVID-19; it may just prevent them from becoming seriously ill with life-threatening diseases such as pulmonary infections, so at the very least, that would take some pressure off the health system.
| Educational resources are available on the PSA website. For more information visit: https://mypsa.org.au/s/education-catalogue |
References
- Prime Minister of Australia. Australia secures onshore manufacturing agreements for two COVID-19 vaccines. 2020. At: pm.gov.au/media/australia-secures-onshore-manufacturing-agreements-two-covid-19-vaccines
- Prime Minister of Australia. Australia secures another 50 million doses of COVID-19 vaccine. 2020. At: https://www.pm.gov.au/media/australia-secures-further-50-million-doses-covid-19-vaccine
- Australian Government Department of Health. Australian COVID-19 vaccination policy. 2020. At: health.gov.au/resources/publications/australian-covid-19-vaccination-policy
- Vette K, Dalton L, Beard F. Pharmacist Vaccination Reporting to the Australian Immunisation Register. May 2020. At: https://bit.ly/32UBZSs
- International Pharmaceutical Federation (FIP). An overview of pharmacy’s impact on immunisation coverage: a global survey. 2020. At: https://www.fip.org/file/4751
- International Pharmaceutical Federation. Governments have a public health duty to expand vaccination coverage through pharmacies, FIP says. 2020. At: https://bit.ly/3fcGEDX
- Department of Health. Ministers. Australia now eligible to purchase COVID-19 vaccine does through COVAX. 2020. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/australia-now-eligible-to-purchase-covid-19-vaccine-doses-through-covax
- Koster E, Philbert D, Bouvy M. Impact of the COVID-19 epidemic on the provision of pharmaceutical care in community pharmacies. Res Soc Admin Pharm 2020. Epub 2020 Jul 2. At: sciencedirect.com/science/article/pii/S1551741120307518?via%3Dihub#
- Patel C, Dalton L, Dey A, et al. Letter: Impact of the COVID-19 pandemic on pharmacist-administered vaccination services. Res Soc Admin Pharm, September 2020:S1551–7411 (20)31049–4. At: sciencedirect.com/science/article/pii/S1551741120310494#






 ‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.
‘We’re increasingly seeing incidents where alert fatigue has been identified as a contributing factor. It’s not that there wasn’t an alert in place, but that it was lost among the other alerts the clinician saw,’ Prof Baysari says.